Azaindazole compounds and methods of use
a technology of azaindazole and compounds, applied in the field of azaindazole compounds and methods of use, can solve the problems of short half-lives once administered, and inability to meet the needs of patients,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
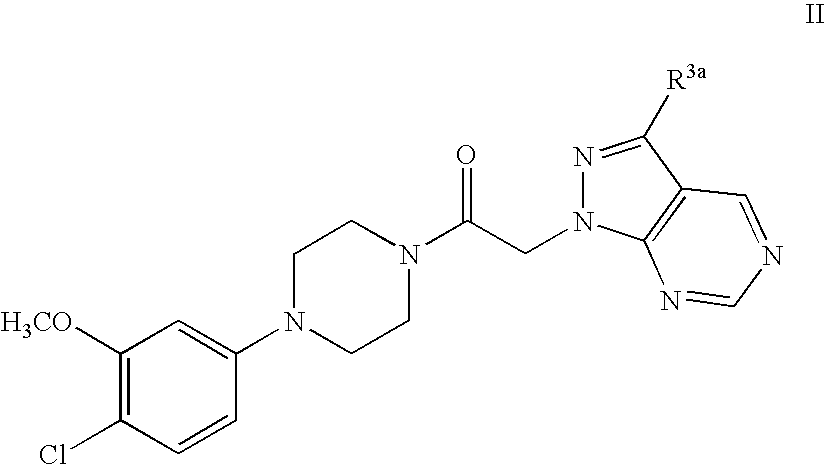
Synthesis of 1-[4-(4-Chloro-3-methoxy-phenyl)-piperazin-1-yl]-2-pyrazolo[3,4-d]pyrimidin-1-yl-ethanone.
[0057]
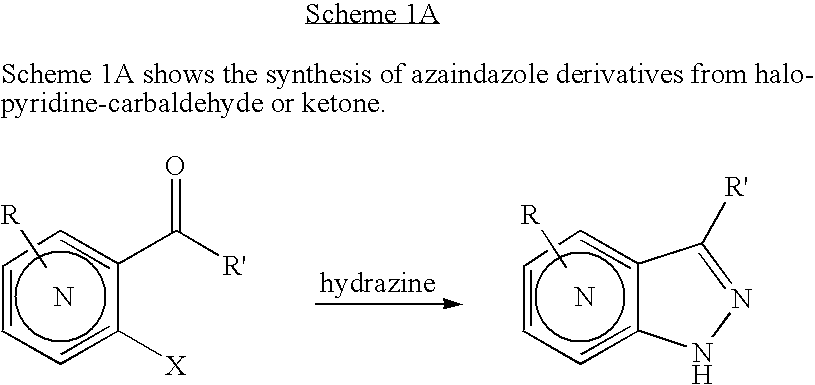
[0058] Preparation of 4-Chloro-1H-pyrazolo[3,4-d]pyrimidine: 4,6-Dichloro-pyrimidine-5-carbaldehyde hydrazine (10 mL, excess), and dioxane (90 mL) are combined at −78° C. in THF. The reaction solution is warmed to rt and stirred for 16 hr. The solvent is evaporated in vacuo to provide a crude residue which is diluted with dichloromethane (600 mL). The organic solution is washed with water (50 mL), brine (50 mL) and dried over anhydrous sodium sulfate. The solvent is removed in vacuo to provide 4-Chloro-1H-pyrazolo[3,4-d]pyrimidine as a yellow powder which is used without further purification.
[0059] Preparation of 1H-Pyrazolo[3,4-d]pyrimidine: 4-Chloro-1H-pyrazolo[3,4-d]pyrimidine Pd(OH)2 and are combined in methanol in a reaction vessel. The reaction vessel is evacuated and backfilled with hydrogen gas and the resultant mixture is stirred at rt for several hours. The result...
example 2
[0061] This example illustrates the method in which compounds of interest (candidate compounds) of the invention can be evaluated for biological activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| humidity | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com