Use of materials for treatment of central nervous system lesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
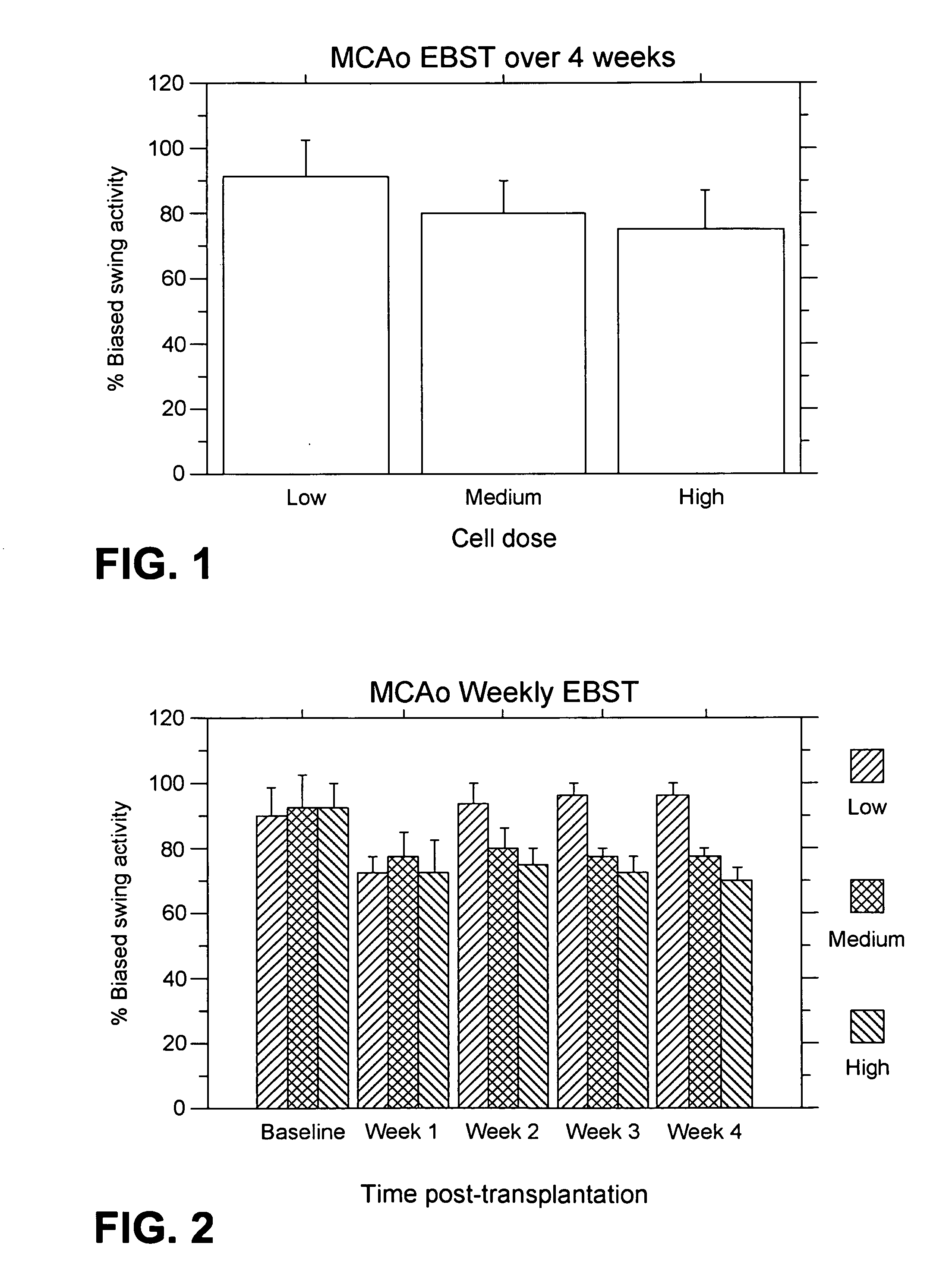
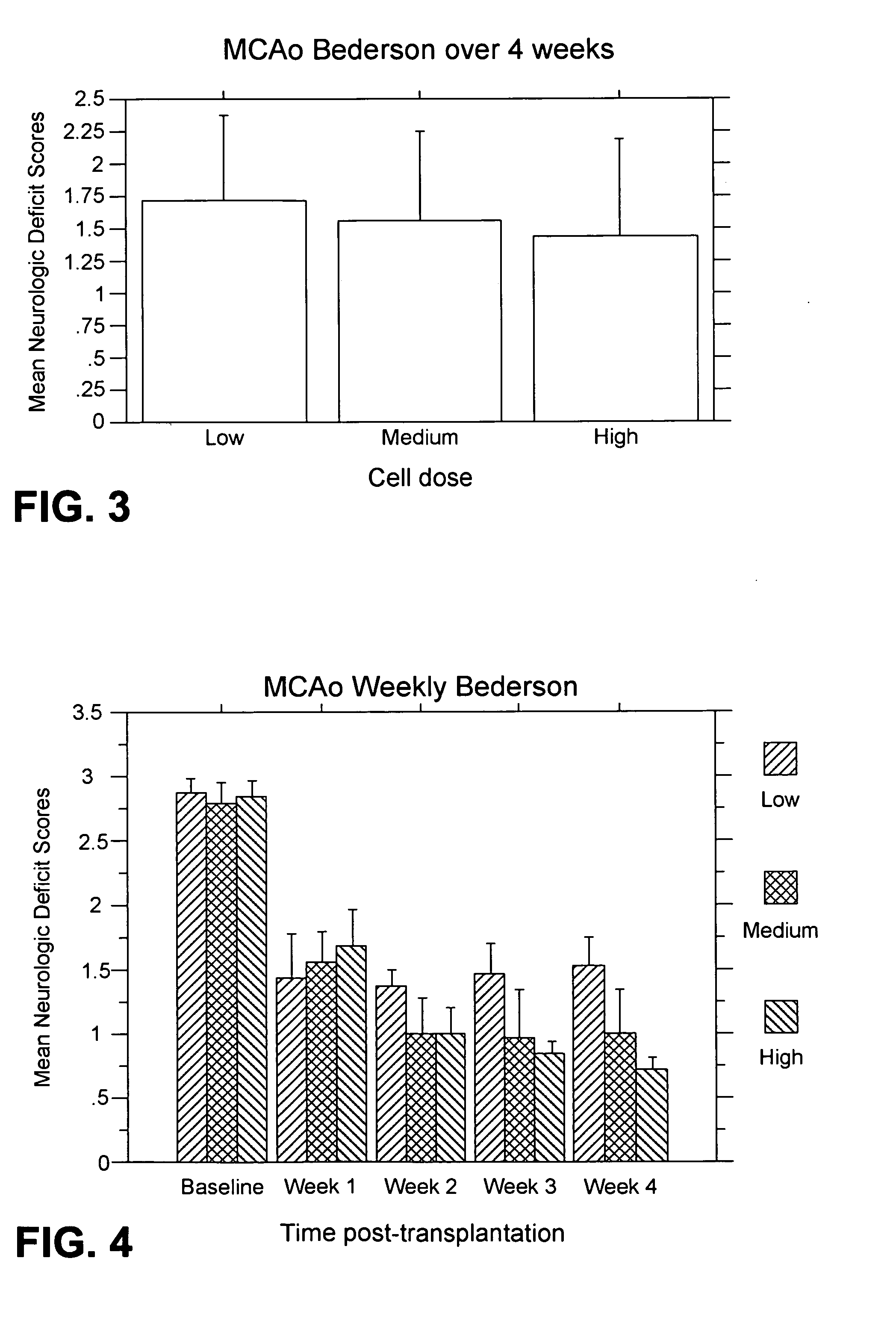
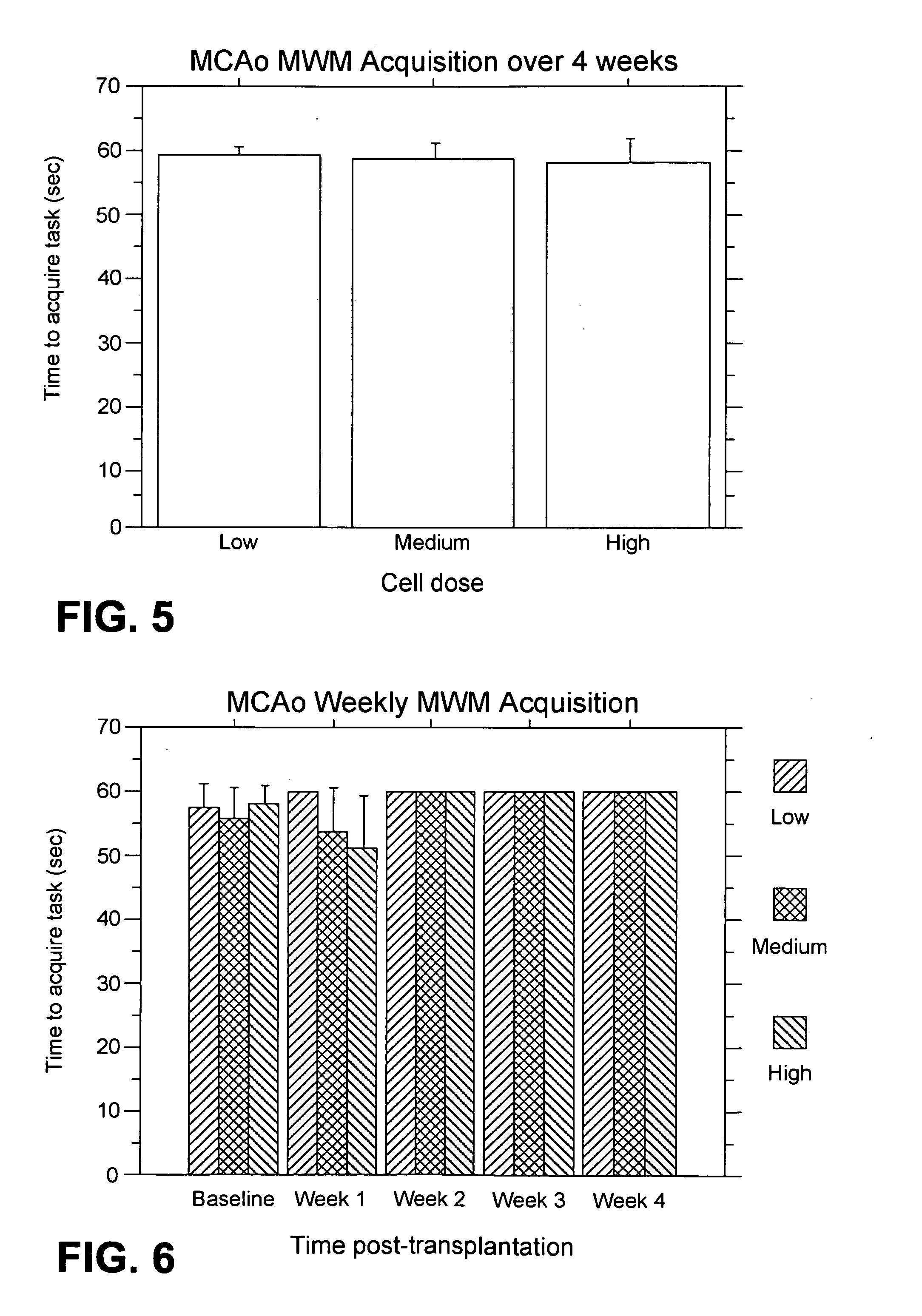
MCAo Results—Weekly for Four Weeks Post-Transplantation
[0202] The test animals underwent the MCAo procedure as described above, and were evaluated weekly for four weeks port-transplantation with the following results.
[0203] EBST: For the weekly testing over the four weeks post-transplantation, overall ANOVA revealed significant main treatment effects (F2,21=57.06, p100,000>40,000) were also observed (p's<0.01). The motor asymmetry was significantly reduced in each of the four weeks post-transplantation compared to baseline (p's<0.0001), with the most robust recovery seen at one week post-transplantation (p's<0.0001), and with stable recovery displayed for the subsequent three weeks post-transplantation. Posthoc tests revealed that the significant reduction in motor asymmetry at 1 week post-transplantation did not differ across the three cell doses, but dose-dependent effects were seen at 2, 3 and 4 weeks post-transplantation (p's<0.05) (FIG. 2).
[0204] Bederson test: For the weekl...
example 2
MCAI Results—Weekly for Four Weeks Post-Transplantation
[0208] The test animals underwent the MCAI procedure as described above, and were evaluated weekly for four weeks post-transplantation with the following results.
[0209] EBST: For the weekly testing over the four weeks post-transplantation, overall ANOVA revealed significant main treatment effects (F2,24=76.30, p100,000>40,000) seen at 1 week post-transplantation (p's<0.05) (FIG. 12).
[0210] Bederson test: For the weekly testing over the four weeks post-transplantation, overall ANOVA revealed significant main treatment effects (F2,24=3.65, p0.005). Posthoc tests revealed that the highest dose of 200,000 cells produced better recovery than the low dose 40,000 cells at 3 and 4 weeks (p's0.05) (FIG. 14).
[0211] MWM Acquisition: For the weekly testing over the four weeks post-transplantation, overall ANOVA revealed no significant main treatment effects (F2,24=5.78-16, p>0.05) (FIG. 15). There appears to be a trend towards longer MW...
example 3
TGI Results—Weekly for Four Weeks Post-Transplantation
[0214] The test animals underwent the TGI procedure as described above, and were evaluated weekly for four weeks port-transplantation with the following results.
[0215] Bederson test: For the weekly testing over the four weeks post-transplantation, overall ANOVA revealed significant main treatment effects (F2,23=47.33, p100,000>40,000) were seen at 4 weeks post-transplantation (p's<0.05) (FIG. 22).
[0216] MWM Acquisition: For the weekly testing over the four weeks post-transplantation, overall ANOVA revealed significant main treatment effects (F2,23=9.88, p<0.001) (FIG. 23). However, this significant treatment effect was achieved only because the highest dose of 200,000 cells produced a transient significant improvement in acquiring the task at 1 week post-transplantation compared to the two other doses of 100,000 and 40,000 cells (p's<0.005). Thereafter, longer acquisition times at 2, 3 and 4 weeks post-transplantation compared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com