Regulation of endogenous gene expression in cells using zinc finger proteins
a technology of endogenous gene expression and recombinant zinc finger protein, which is applied in the direction of fusion polypeptide, peptide/protein ingredients, vectors, etc., can solve the problems of not being able to demonstrate high target efficacy or high specificity in vivo, and the method is labor-intensive and labor-intensive. , to achieve the effect of modulating the expression of endogenous cellular genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0228] The following examples are provided by way of illustration only and not by way of limitation. Those of skill in the art will readily recognize a variety of noncritical parameters that could be changed or modified to yield essentially similar results.
example i
Design and Testing of ZFPs Targeted to the Human VEGF Gene
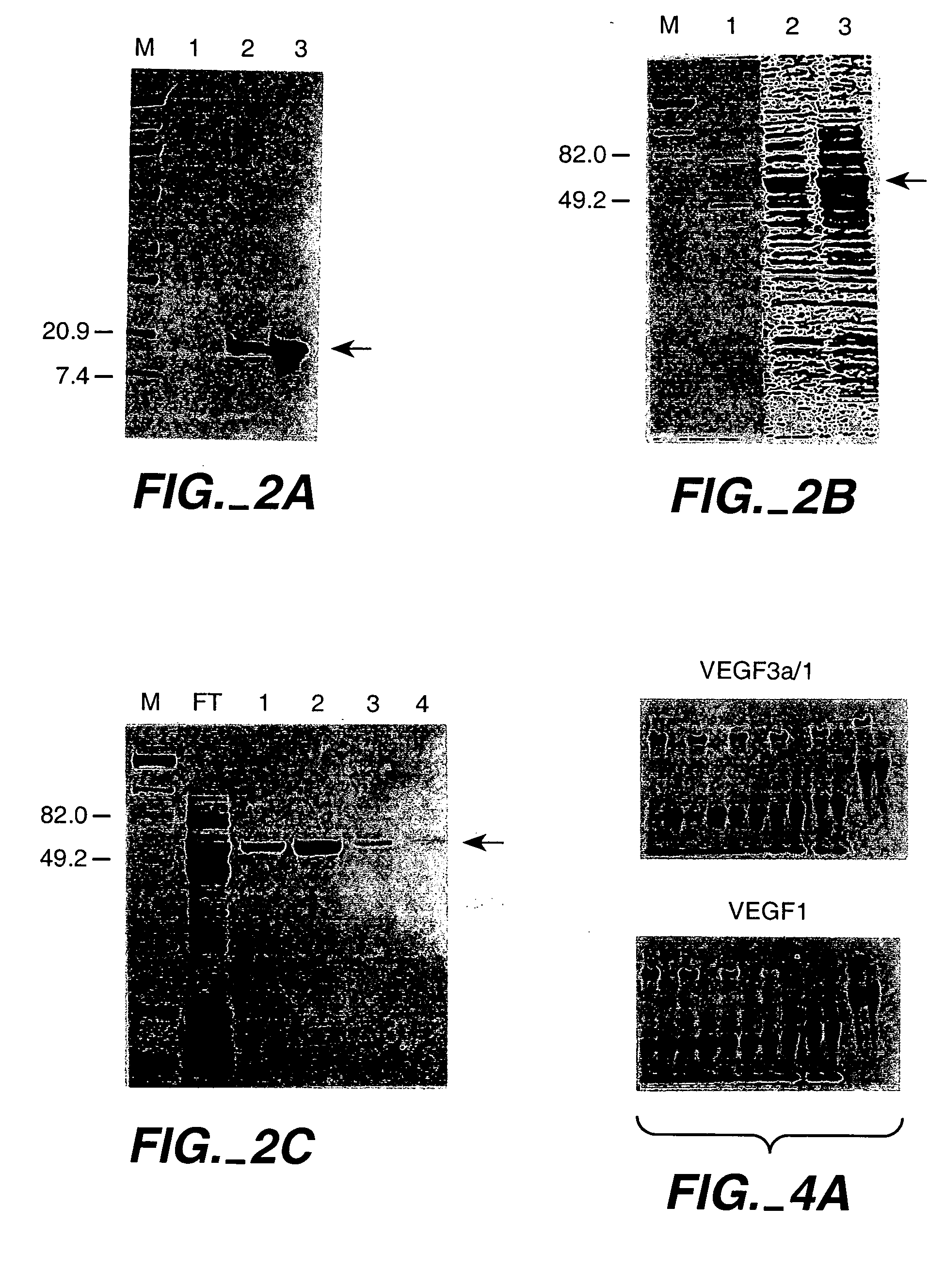
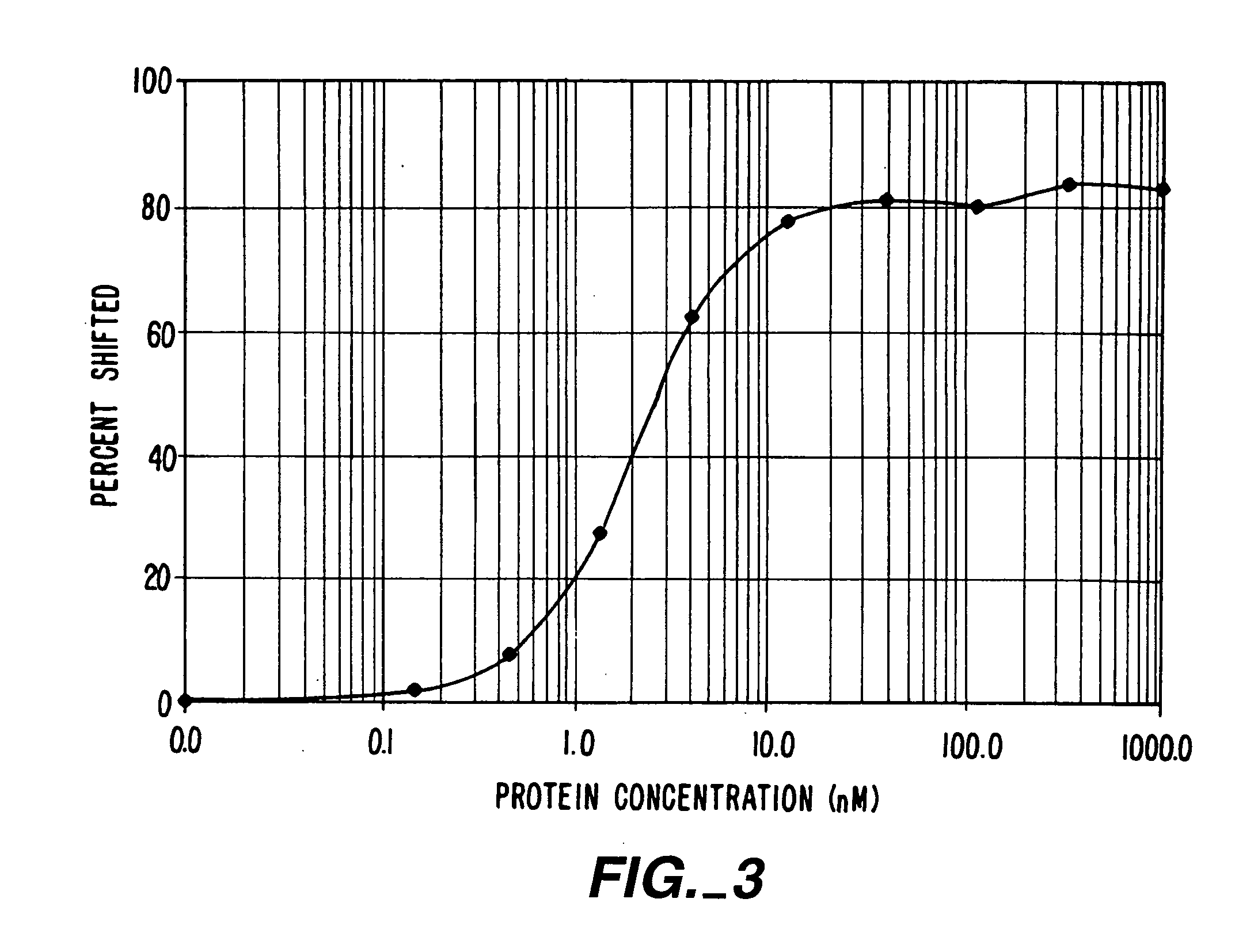
[0229] This first Example demonstrates the construction of ZFPs designed to recognize DNA sequences contained in the promoter of the human vascular endothelial growth factor (VEGF) gene. VEGF is an approximately 46 kDa glycoprotein that is an endothelial cell-specific mitogen induced by hypoxia. VEGF has been implicated in angiogenesis associated with cancer, various retinopathies, and other serious diseases. The DNA target site chosen was a region surrounding the transcription initiation site of the gene. The two 9 base pair (bp) sites chosen are found within the sequence agcGGGGAGGATcGCGGAGGCTtgg, where the upper-case letters represent actual 9-bp targets. The protein targeting the upstream 9-bp target was denoted VEGF1, and the protein targeting the downstream 9-bp target was denoted VEGF3a. The major start site of transcription for VEGF is at the T at the 3′ end of the first 9-bp target, which is underlined in the sequen...
example ii
Linking ZFPs to Bind an 18-bp Target in the Human VEGF Gene
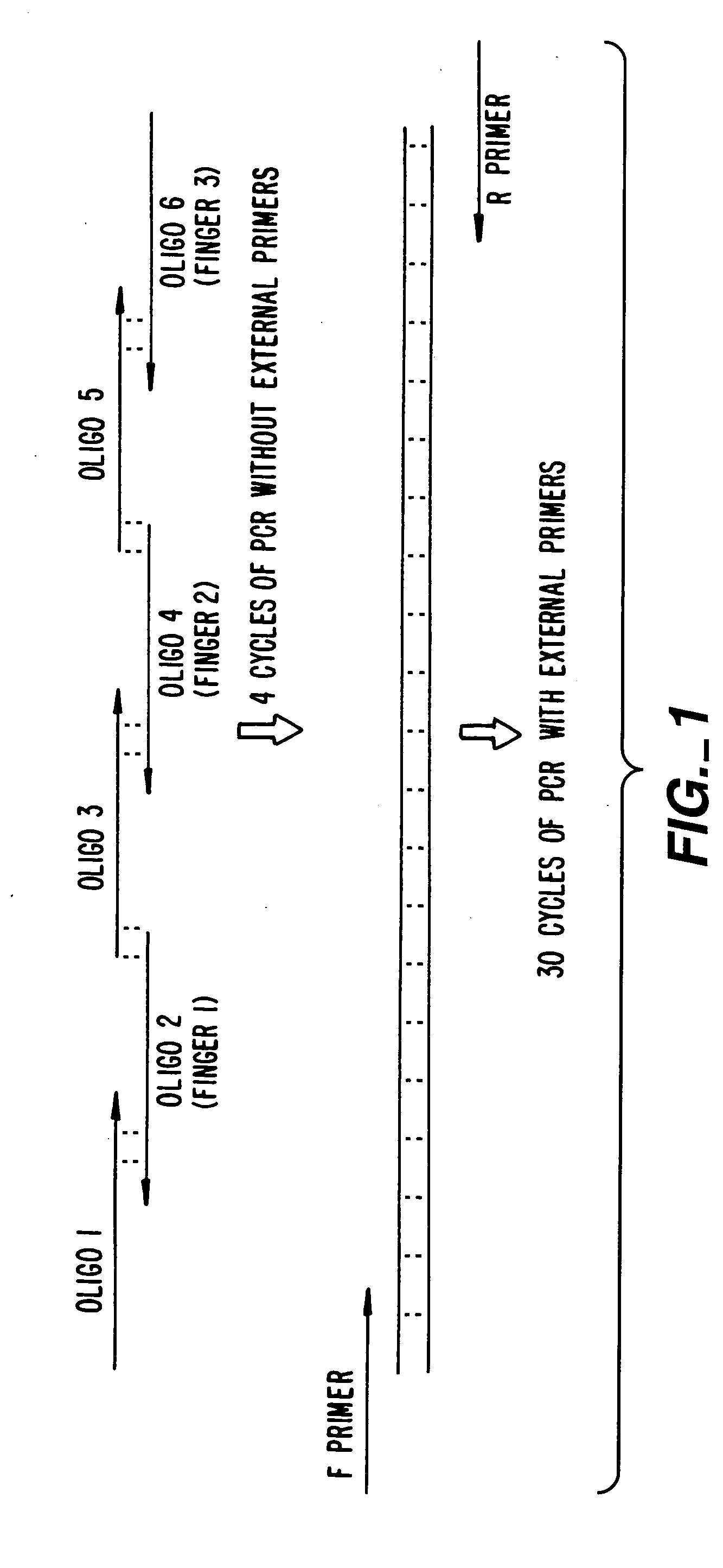
[0248] An important consideration in ZFP design is DNA target length. For random DNA, a sequence of n nucleotides would be expected to occur once every 0.5×4n base-pairs. Thus, DNA-binding domains designed to recognize only 9 bp of DNA would find sites every 130,000 bp and could therefore bind to multiple locations in a complex genome (on the order of 20,000 sites in the human genome). 9-bp putative repressor-binding sequences have been chosen for VEGF in the 5′ UTR where they might directly interfere with transcription. However, in case zinc finger domains that recognize 9-bp sites lack the necessary affinity or specificity when expressed inside cells, a larger domain was constructed to recognize 18 base-pairs by joining separate three-finger domains with a linker sequence to form a six-finger protein. This should ensure that the repressor specifically targets the-appropriate sequence, particularly under conditions where o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com