Methods and compositions for derepression of IAP-inhibited caspase
a technology of iapin-inhibited caspase and composition, which is applied in the field ofmolecular medicine, can solve the problems of complex death process, defective regulation of programmed cell death, and defects in programmed cell death, and achieve the effects of promoting apoptosis, reducing the severity of a pathology, and reducing the level of apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Identification of Derepressors of an IAP-Inhibited Caspase from Hexapeptide Libraries
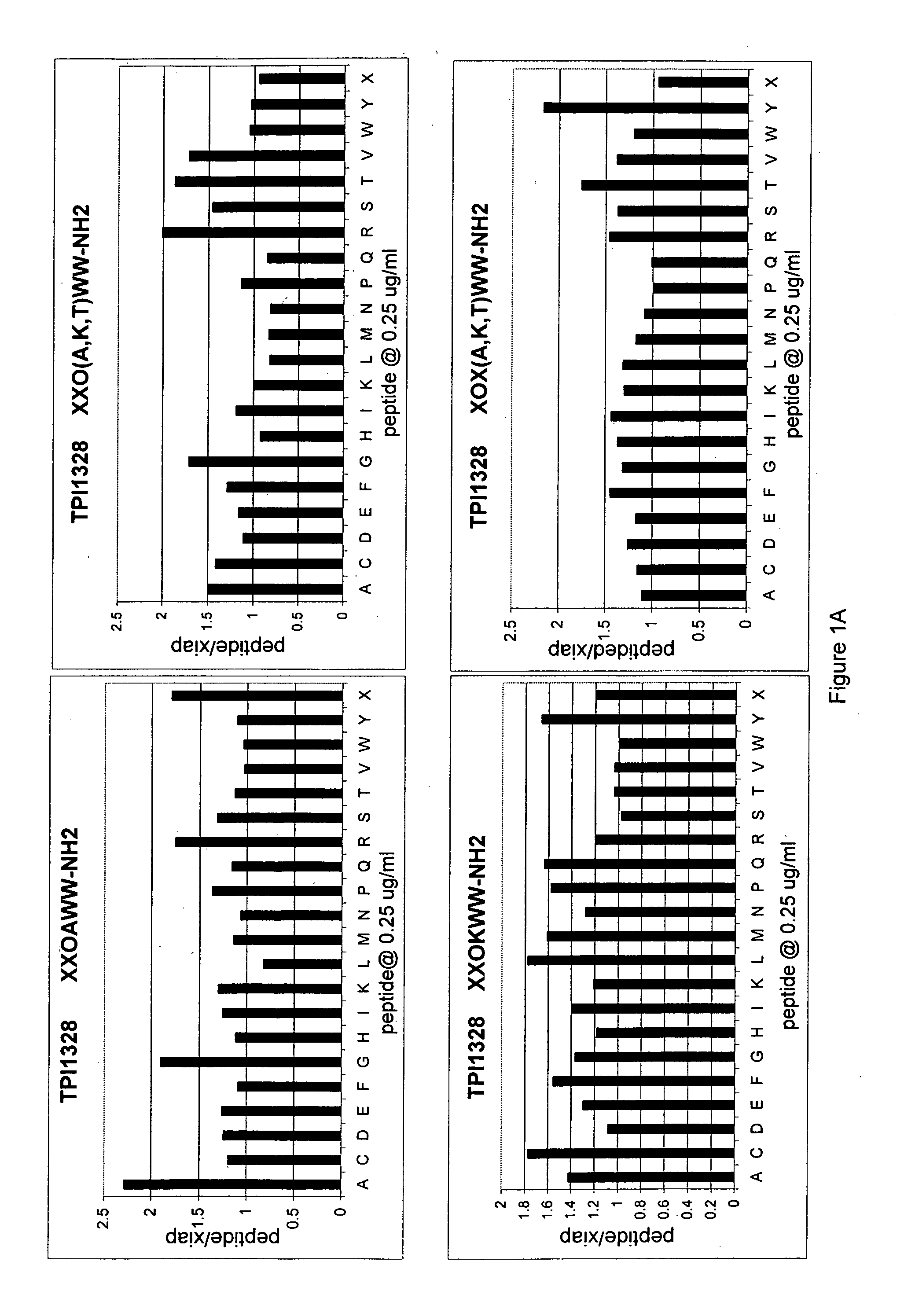
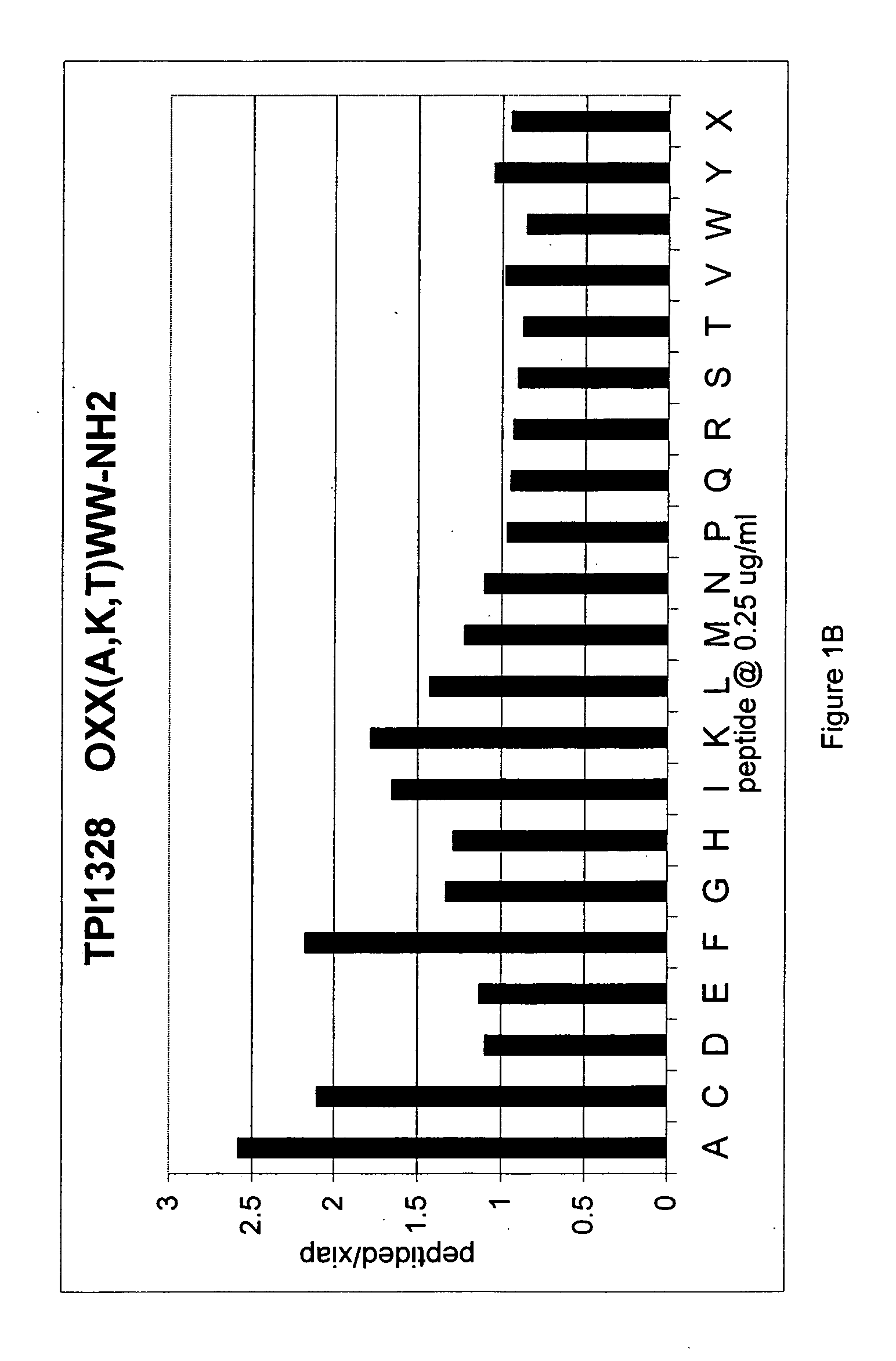
[0226] This example demonstrates an IAP derepression assay. This Example further demonstrates a positional-scanning approach to identifying agents that are capable of derepressing an IAP-inhibited caspase.
[0227] The DCR390 library consisting of 120 mixtures of hexapeptides was synthesized using methods known in the art as described in R. Houghten et al. PCT / US91 / 08694 and U.S. Pat. No. 5,556,762. Each mixture was made up of a population of hexapeptides all of which had the same amino acid at a defined position and any combination of the 20 essential amino acids at the remaining 5 positions. Each mixture is identified by the position number where the defined amino acid occurs (numbered from 1 to 6 going from the amino-terminus to carboxy-terminus of the hexapeptide) and the identity of the defined amino acid. Thus, as shown in the first column of Table I, the mixture having a tryptophan at position...
example ii
Identification of Derepressors of an IAP-Inhibited Caspase from the TPI1332 and TPI1352 Individual Tetrapeptide Libraries
[0236] This Example demonstrates identification of agents from the TPI1332 and TPI1352 tetrapeptide libraries that are capable of derepressing an XIAP-inhibited caspase-3.
[0237] The TPI1332 and TPI1352 tetrapeptide libraries were synthesized identically with the exception that the formyl protecting groups on tryptophan were removed by different procedures. The deprotection step used for the TPI1332 library was less complete leaving the possibility that some of the tryptophan residues present on candidate compounds used in the screen retained formyl protecting groups. The deprotecting chemistry used for the TPI1352 library was substantially complete, however resulted in the formation of polymeric structures for a subset of the species in the library.
[0238] Candidates from the TPI1332 and TPI1352 libraries were screened using the derepression assay described in E...
example iii
Identification of Individual Peptide Derepressors of an IAP-Inhibited Caspase from the TPI792 Library
[0240] This Example demonstrates identification of agents from the TPI792 library that are capable of derepressing an XIAP-inhibited caspase-3.
[0241] The TPI792 library is based on a tetrapeptide backbone. The species of the TPI792 library were screened in the derepression assay described in Example I. A list of derepressors of XIAP-inhibited caspase-3 identified from the TPI792 library is provided in Table V. Structures for the TPI792 core peptides that were tested are shown in FIG. 20.
TABLE VLCAgentPos 1Pos 2Pos 3Pos 4μg / ml792-3D-NalLysεFmocL-pClPheLys-εFmoc2792-9D-NalD-pClPheL-pClPheLys-εFmoc10792-15D-NalL-NalL-pClPheD-Lys-εFmoc2792-17D-NalL-NalD-Lys (Fm)Lys-εFmoc2792-19L-ThiAlaLys-εFmocD-NalLys-εFmoc2792-22L-ThiAlaLys-εFmocL-pClPheD-PFPhe2792-27L-ThiAlaD-pClPheL-pClPheLys-εFmoc2792-33L-ThiAlaL-NalL-pClPheLys-εFmoc0.4792-35L-ThiAlaL-NalD-Lys (Fm)D-Lys-εFmoc2
[0242] The dose res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com