Prevention and treatment of amyloidogenic disease
a technology of amyloidogenic disease and amyloid aggregation, which is applied in the field of immunology and medicine, can solve the problem of implausible therapeutic benefi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
I. Prophylactic Efficacy of Aβ Against AD
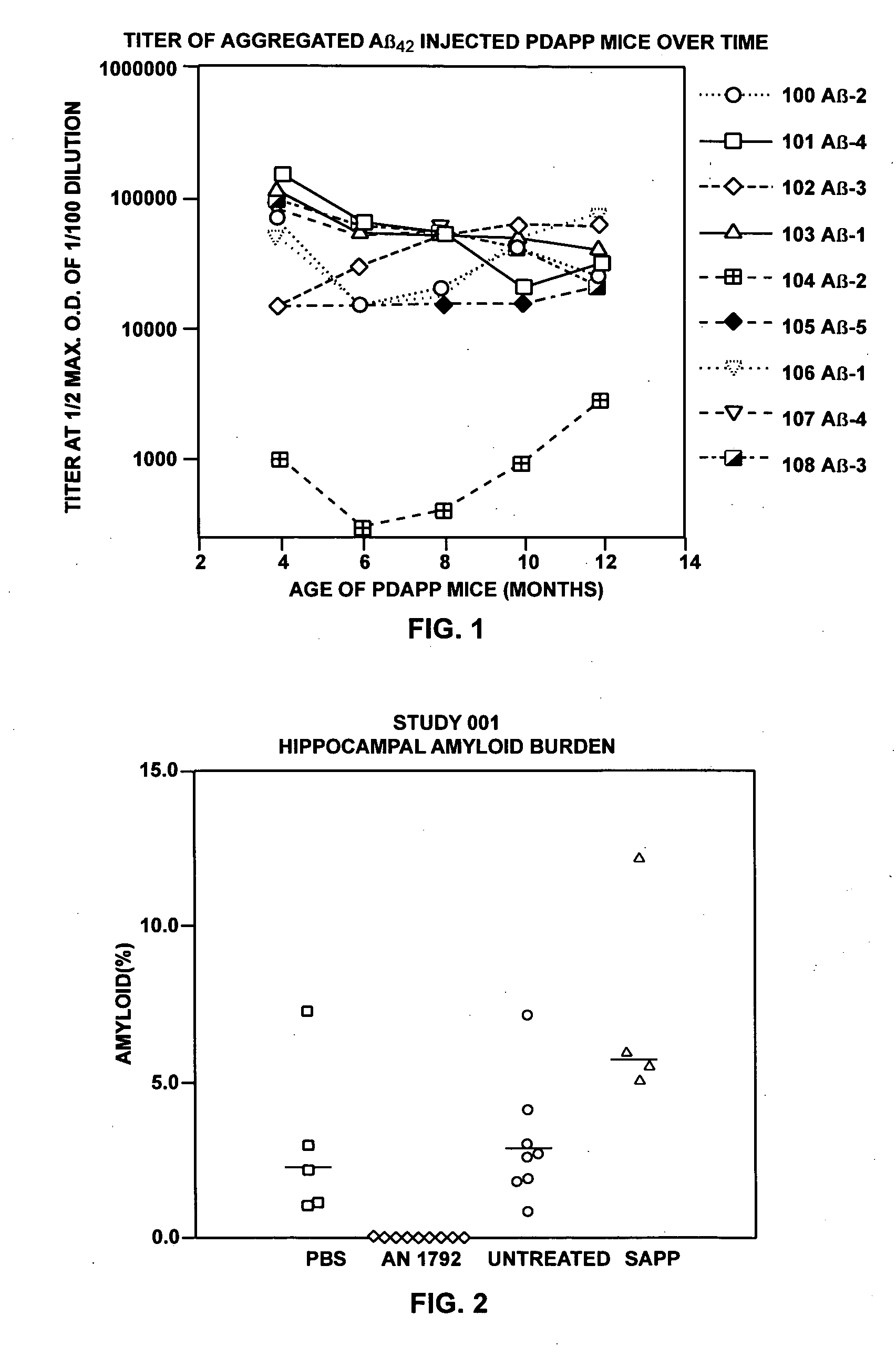
[0198] These examples describe administration of Aβ42 peptide to transgenic mice overexpressing APP with a mutation at position 717 (APP717V→F) that predisposes them to develop Alzheimer's-like neuropathology. Production and characteristics of these mice (PDAPP mice) is described in Games et al., Nature, supra. These animals, in their heterozygote form, begin to deposit Aβ at six months of age forward. By fifteen months of age they exhibit levels of Aβ deposition equivalent to that seen in Alzheimer's disease. PDAPP mice were injected with aggregated Aβ42 (aggregated Aβ42) or phosphate buffered saline. Aggregated Aβ42 was chosen because of its ability to induce antibodies to multiple epitopes of Aβ.
[0199] A. Methods
[0200] 1. Source of Mice
[0201] Thirty PDAPP heterogenic female mice were randomly divided into the following groups: 10 mice to be injected with aggregated Aβ42 (one died in transit), 5 mice to be injected with PBS / adjuvant or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com