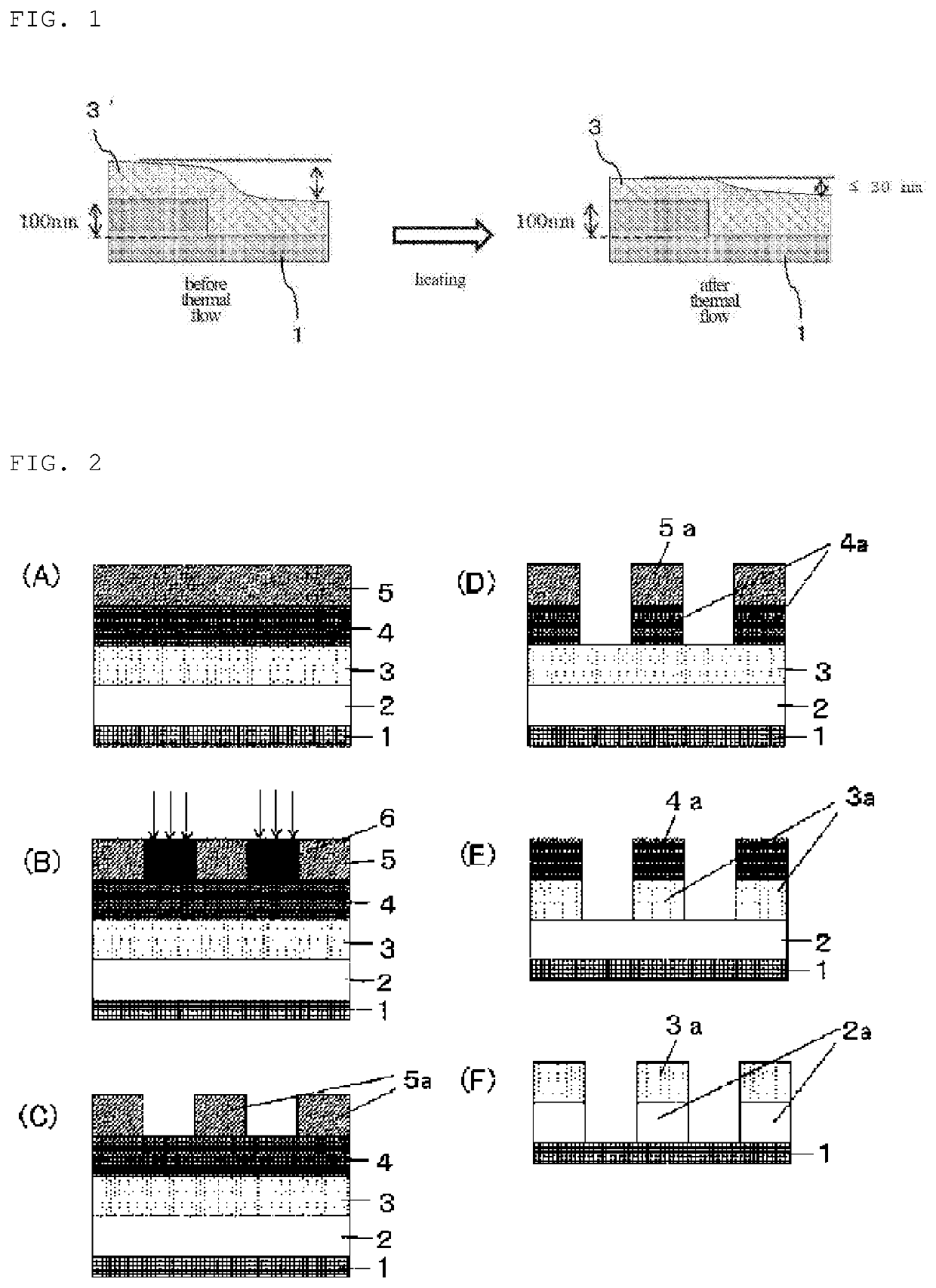
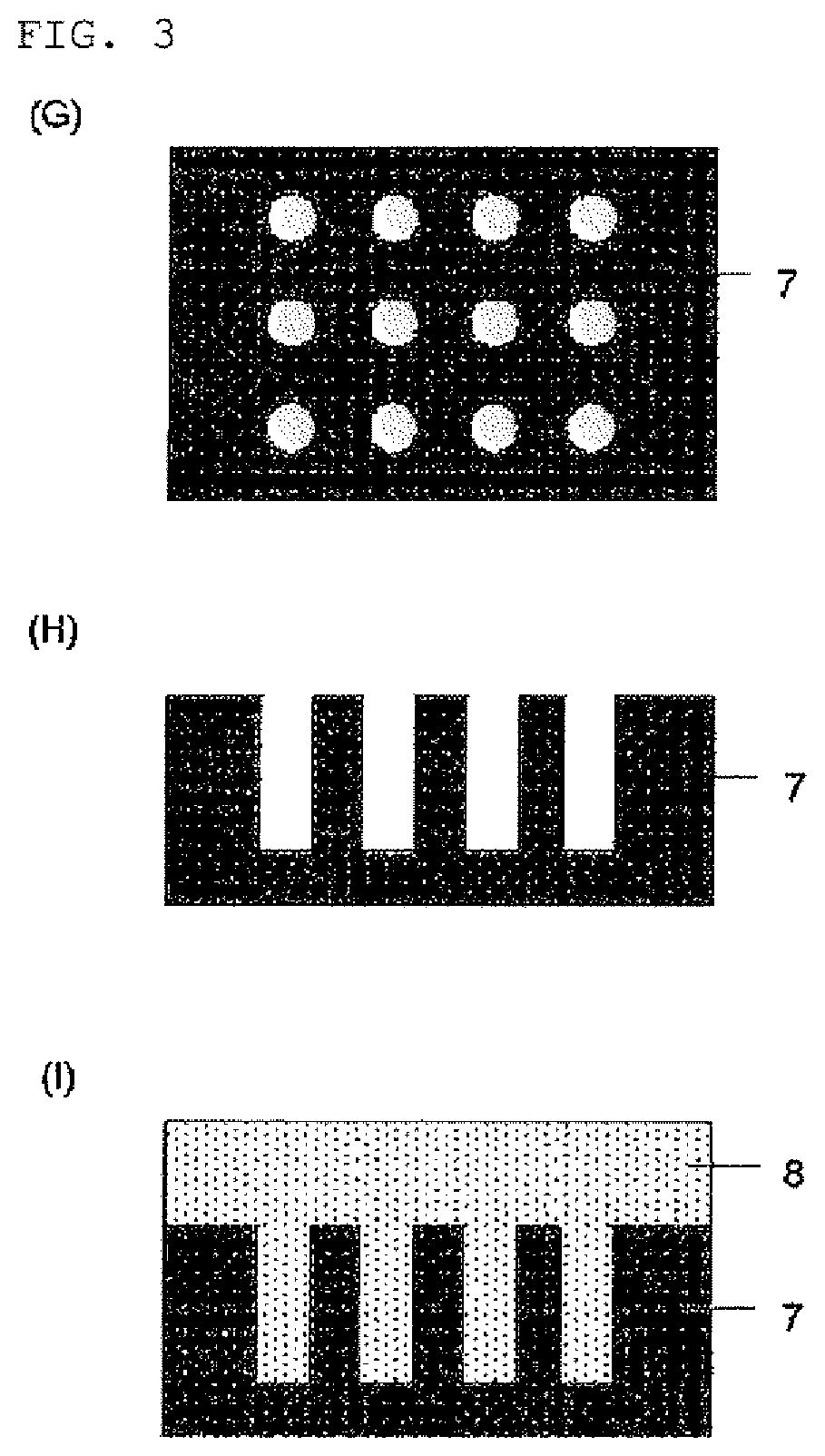
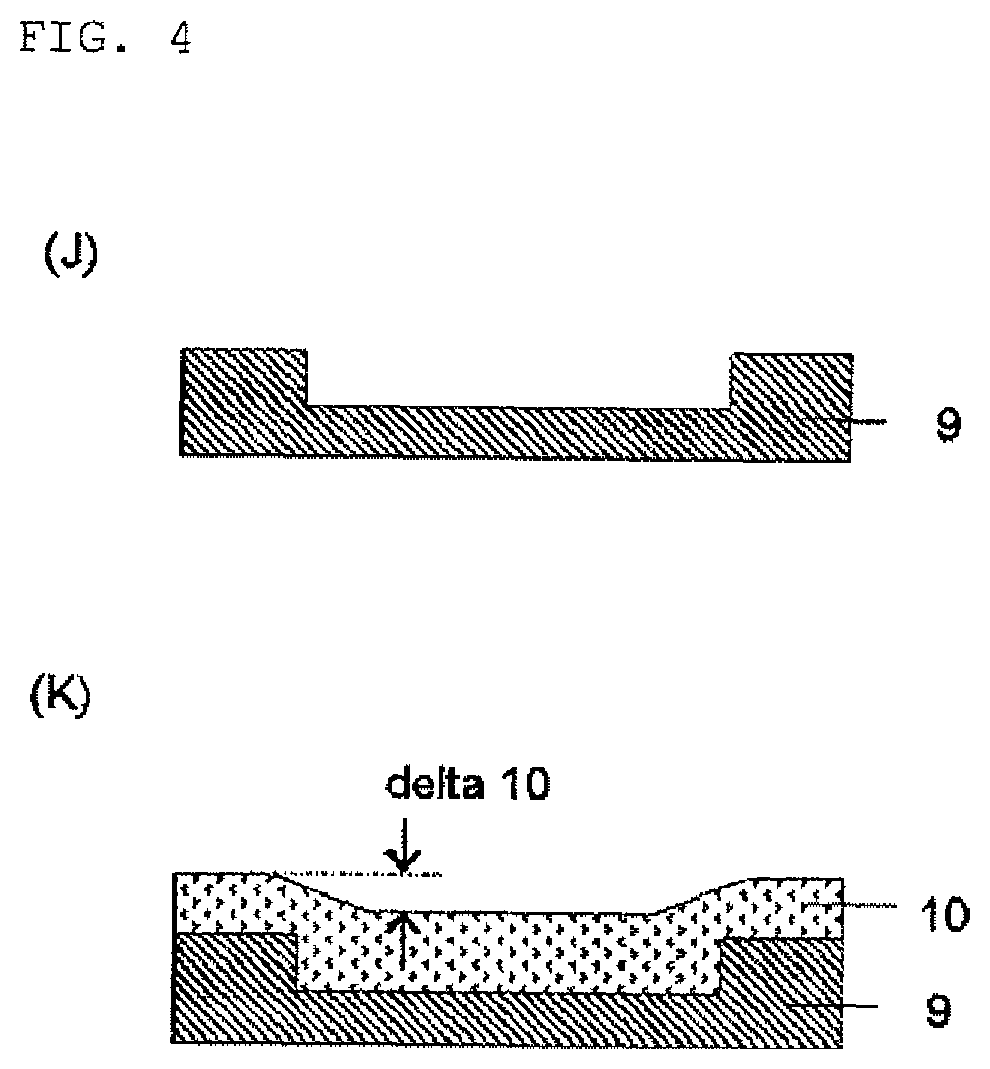
Compound and composition for forming organic film
a composition and organic film technology, applied in the field of compound and composition for forming organic films, can solve the problems of not having a complete etching selectivity between photoresist films, the production process of semiconductor devices is approaching the inherent limit, and it is difficult to produce semiconductor devices in a good yield. , to achieve the effect of excellent gap filling/planarizing characteristics, excellent heat resistance, and superior gap filling and planarizing characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
[Synthesis Example 1] Synthesis of Compound (A1)
[0157]
[0158]A mixture of 7.7 g of Diol (B1), 3.0 g of potassium carbonate, and 40 g of N,N-dimethylformamide was heated to 55° C. To the mixture, 2.9 g of 1-bromo-2-butyne was slowly added dropwise, and this was stirred with heating at 55° C. for 20 hours. After cooling to room temperature, 150 g of methyl isobutyl ketone was added thereto. This was washed with water and concentrated in vacuum, and then precipitated with methanol. The yielded solid was filtered off, washed with methanol, and dried in vacuum to give 8.7 g of the object (A1). The following are analytical results of IR and 1H NMR for the synthesized Compound (A1).
[0159]IR (D-ATR): ν=3057, 3029, 2954, 2916, 2867, 1601, 1494, 1476, 1446, 1217, 1005, and 819 cm−1
[0160]1H NMR (600 MHz, DMSO-d6) δ (ppm): 7.94 (d, J=7.3 Hz, 4H), 7.71 (d, J=9.2 Hz, 2H), 7.60 (d, J=9.2 Hz, 2H), 7.51-7.45 (m, 10H), 7.41-7.38 (m, 4H), 7.31-7.28 (m, 6H), 7.28-7.15 (m, 6H), 7.08-7.06 (m, 2H), 4.80 (...
synthesis example 2
[Synthesis Example 2] Synthesis of Compound (A1)
[0162]
[0163]A mixture of 7.7 g of Diol (B1), 3.0 g of potassium carbonate, and 40 g of N,N-dimethylformamide was heated to 55° C. To the mixture, 3.3 g of 80% propargyl bromide toluene solution was slowly added dropwise, and this was stirred with heating at 55° C. for 14 hours. After cooling to room temperature, 150 g of toluene was added thereto. This was washed with water and concentrated in vacuum to give 8.4 g of Propargyl derivative (52).
[0164]Then, under an N2 atmosphere, to a mixture of 8.4 g of Propargyl derivative (52) and 60 mL of tetrahydrofuran cooled to −30° C., 10 mL of 2.65 M n-butyl lithium hexane solution was added, and this was stirred at −30° C. for 1 hour. To this, 4.4 g of dimethyl sulfate was added. This was allowed to gradually raise the temperature to room temperature, heated to 60° C., and stirred for 6 hours. After cooling to room temperature, dilute hydrochloric acid wad added to stop the reaction, and 100 g ...
synthesis example 3
[Synthesis Example 3] Synthesis of Compound (A2)
[0168]To a mixture of 8.78 g of 4,4′-dihydroxybenzophenone and 50 mL of acetone, 17 g of potassium carbonate was added, and 7.9 mL of 1-bromo-2-butyne was added dropwise. The reaction mixture was heated and was heated to reflux overnight. After cooling to room temperature, followed by filtration, the filtrate was concentrated in vacuum. Then, mixed solvent of methanol:Chloroform=9:1 was added thereto, and yielded solid was filtered off to give 10.6 g of Intermediate (B3) shown by the following formula. The following are analytical results of 1H NMR for the synthesized Intermediate (B3).
[0169]
[0170]1H NMR (400 MHz, CDCl3) δ (ppm): 7.81 (d, Ar—H, 4H), 7.05 (d, Ar—H, 4H), 4.75 (s, CH2, 4H), 1.90 (s, CH3, 6H).
[0171]Then, under an N2 atmosphere, to a mixture of 5.44 g of 4,4′-dibromobiphenyl and 50 mL of tetrahydrofuran cooled to −70° C., 23.92 mL of 1.6 M n-butyl lithium hexane solution was added, and this was stirred at −40° C. for 1 hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com