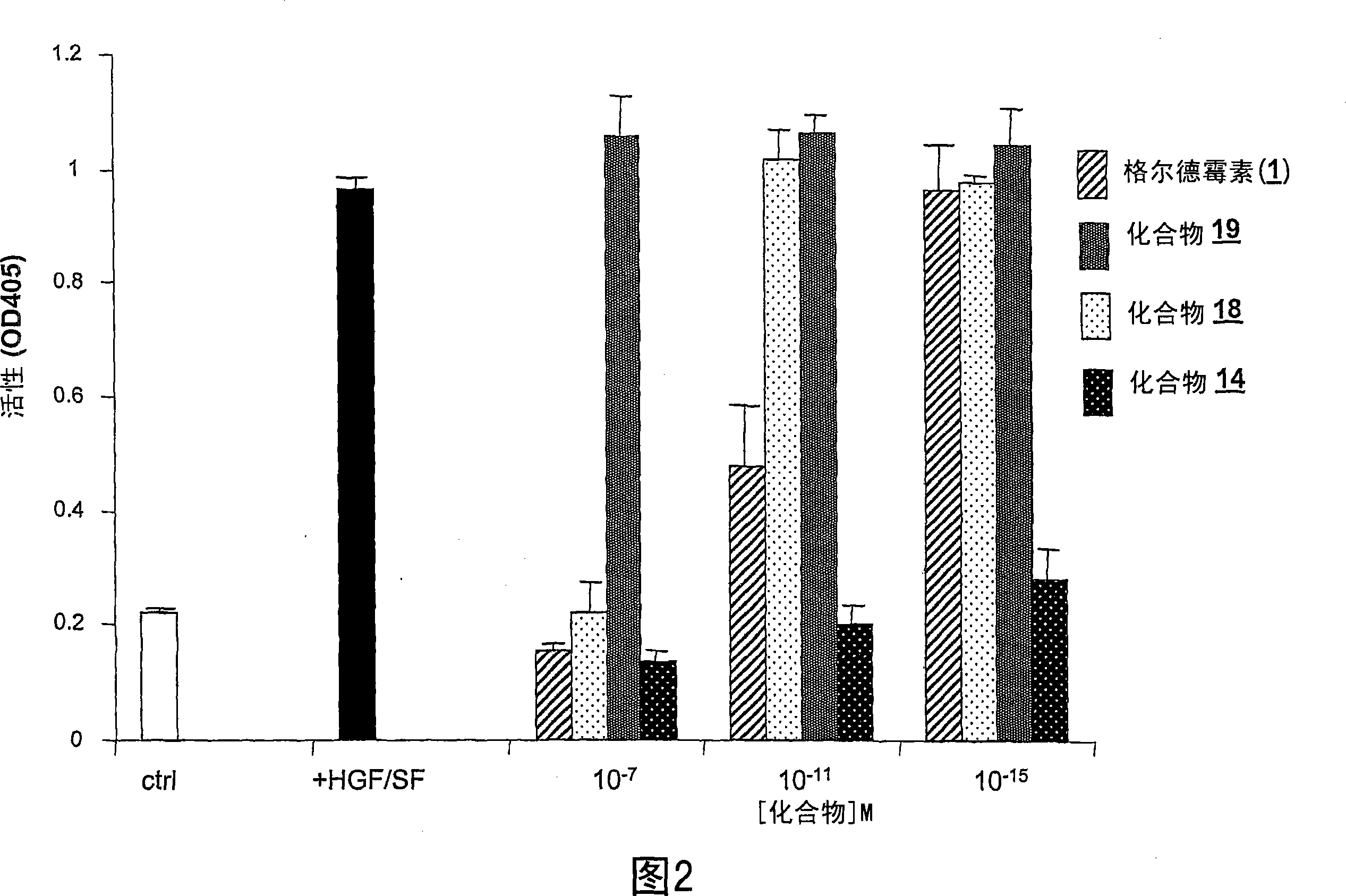
Geldanamycin and derivatives inhibit cancer invasion and identify novel targets
A compound, cancer cell technology, applied in the field of cancer pharmacology, which can solve the problem of not providing anti-tumor effect and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-19
[0235] Synthesis and / or Characterization of Geldanamycin and Derivatives
[0236] general method. Melting points are unmodified. Infrared spectra were recorded on a Matton Galaxy Series FTIR 3000 spectrophotometer. UV-vis spectra were recorded on a Hitachi U-4001 spectrophotometer. Recorded on a Varian Inova-600, UnityPlus-500, VRX-500 or VRX-300 spectrometer 1 H and 13 CNMR spectrum. The numbering used in all assignments is based on the GA ring system (Sasaki, K et al., J. Ain. Clzem. Soc. 92:7591 (1970)), unless otherwise stated). Mass spectra were performed by MSU Mass Spectrometry Facility. GA and macbecinII were provided by National Cancer Institutes. Macbecin I was synthesized from macbecin II by a published procedure (Muroi, M et al., 1980). Radicicol is commercially available (Sigma-Aldrich). Anhydrous solvents were purified using standard methods.
Embodiment 1
[0238] (+)-Geldanamycin (1)
[0239] IR(CH 2 Cl 2 )(cm -1 )3535, 3421, 3364, 3060, 2989, 2968, 1733, 1690, 1650, 1603, 1500, 1367, 1284, 1262, 1193, 1135, 1098, 1054; 1 H NMR (CDCl 3 , 500MHz, assigned with COZY) δ8.69 (s, IH) (22-NH), 7.27 (s, 1H) (19-H), 6.92 (bd, J = 11.5Hz, 1H) (3-H) , 6.55 (ddd, J=11.5, 11.0, 1.0Hz, 1H) (4-H), 5.86 (dd, J=11.0, 10.0Hz, 1H) (5-H), 5.80 (bd, J=9.5Hz, 1H)(9-H), 5.17(s,1H)(7-H), 4.77(bs,2H)(7-O2CNH 2 ), 4.29 (bd, J=10.0Hz, 1H) (6-H), 4.10 (s, 3H) (17-OCH 3 ), 3.51(ddd, J=9.0, 6.5, 2.0Hz, 1H)(H-H), 3.37(ddd, J=9.0, 3.0, 3.0Hz, 1H)(12-H), 3.34(s, 3H)(6 - or 12-OCH 3 ), 3.27(s, 3H) (6- or 12-OCH 3 ), 3.03 (bd, J=6.5Hz, IH) (11-OH), 2.76 (dqd, J=9.5, 7.0, 2.0Hz, 1H) (10-H), 2.50-2.39 (m, 2H) (15 -H and H 1 ), 2.00(bs,3H)(2-CH 3 ), 1.81-1.70 (m, 2H) (13-H and H 1 ), 1.77 (d, J=1.0Hz, 3H) (8-CH 3), 1.68-1.60 (m, 1H) (14-H), 0.97-0.93 (m, 6H) (10- and 14-CH 3 ); (Sasaki et al., 1970, supra; Organic Synthesis, Cumulative Volume 4, 433,...
Embodiment 2
[0241] 17-allylamino-17-desmethoxygeldanamycin (4)
[0242] (Schnur, RC et al., 1995a, 1995b) Mix (+)-geldanamycin (5.1 mg, 9.0 mol) with allylamine (10.0 μl, 0.13 mmol) in chloroform (1.5 ml) at room temperature Stir. Based on complete conversion of GA shown by thin layer chromatography (18 h), the mixture was washed with brine, dried over anhydrous sodium sulfate, and concentrated. The product was isolated as a purple solid (5.3 mg, 99%) by flash column chromatography on silica gel (n-hexane / ethyl acetate). IR(KBr)(cm -1 ) 3464, 3333, 2958, 2929, 2825, 1728, 1691, 1652, 1571, 1485, 1372, 1323, 1189, 1101, 1057; UV (95% EtOH) (nm) 332 (ε = 2.0 × 104); 1H NMR (CDCl 3 , 500MHz) δ9.14(s, 1H), 7.28(s, 1H), 6.93(bd, J=11.5Hz, 1H), 6.56(bdd, J=11.5, 11.0Hz, 1H), 6.38(bt, J =6.0Hz, 1H), 5.94-5.81(m, 3H), 5.30-5.24(m, 2H), 5.17(s, 1H), 4.82(bs, 2H), 4.29(bd, J=10.0Hz, 1H) , 4.21 (bs, 1H), 4.18-4.08 (m, 2H), 3.55 (ddd, J=9.0, 6.5, 2.0Hz, 1H), 3.43 (ddd, J=9.0, 3.0, 3.0Hz, 1H), 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com