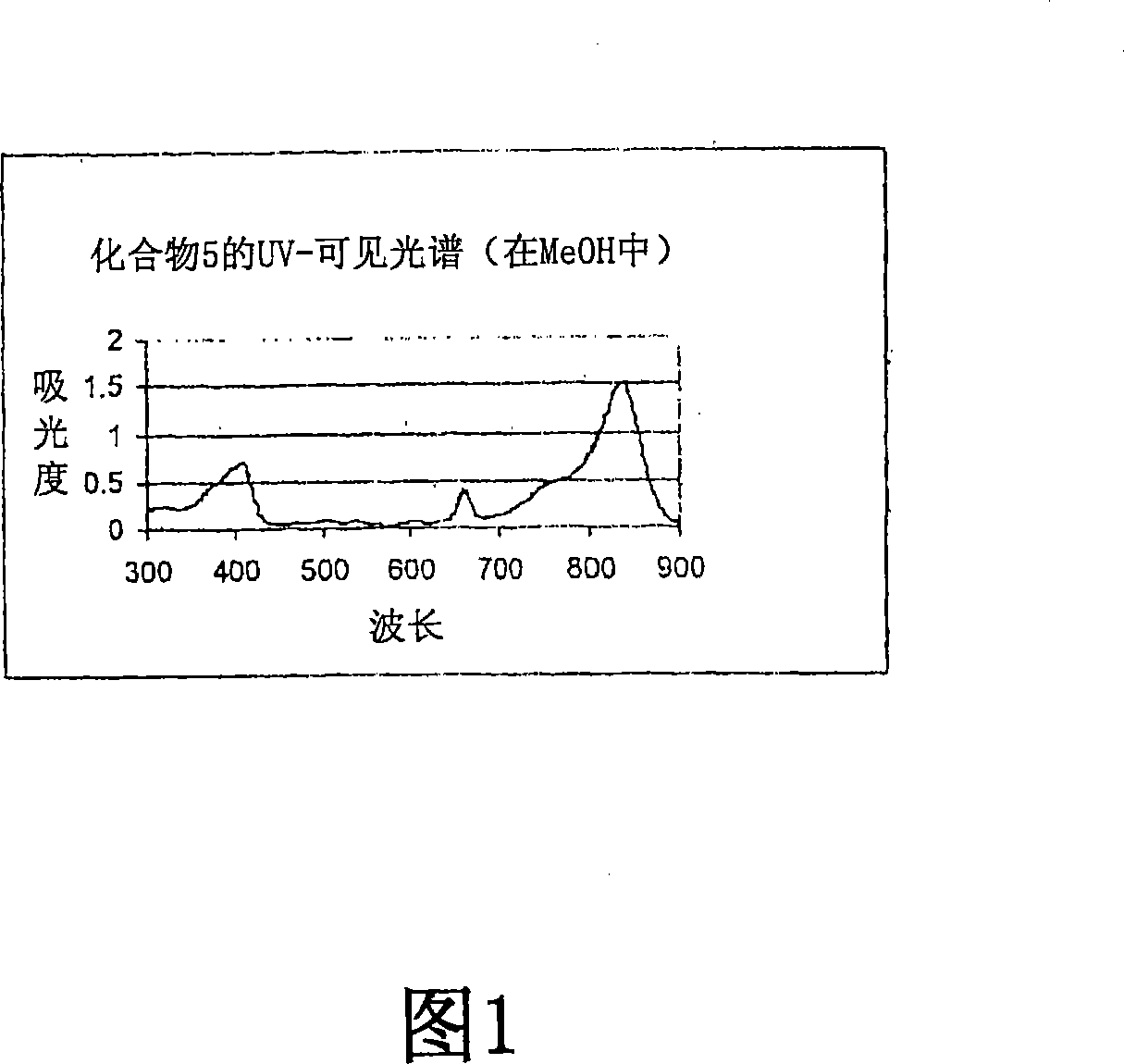
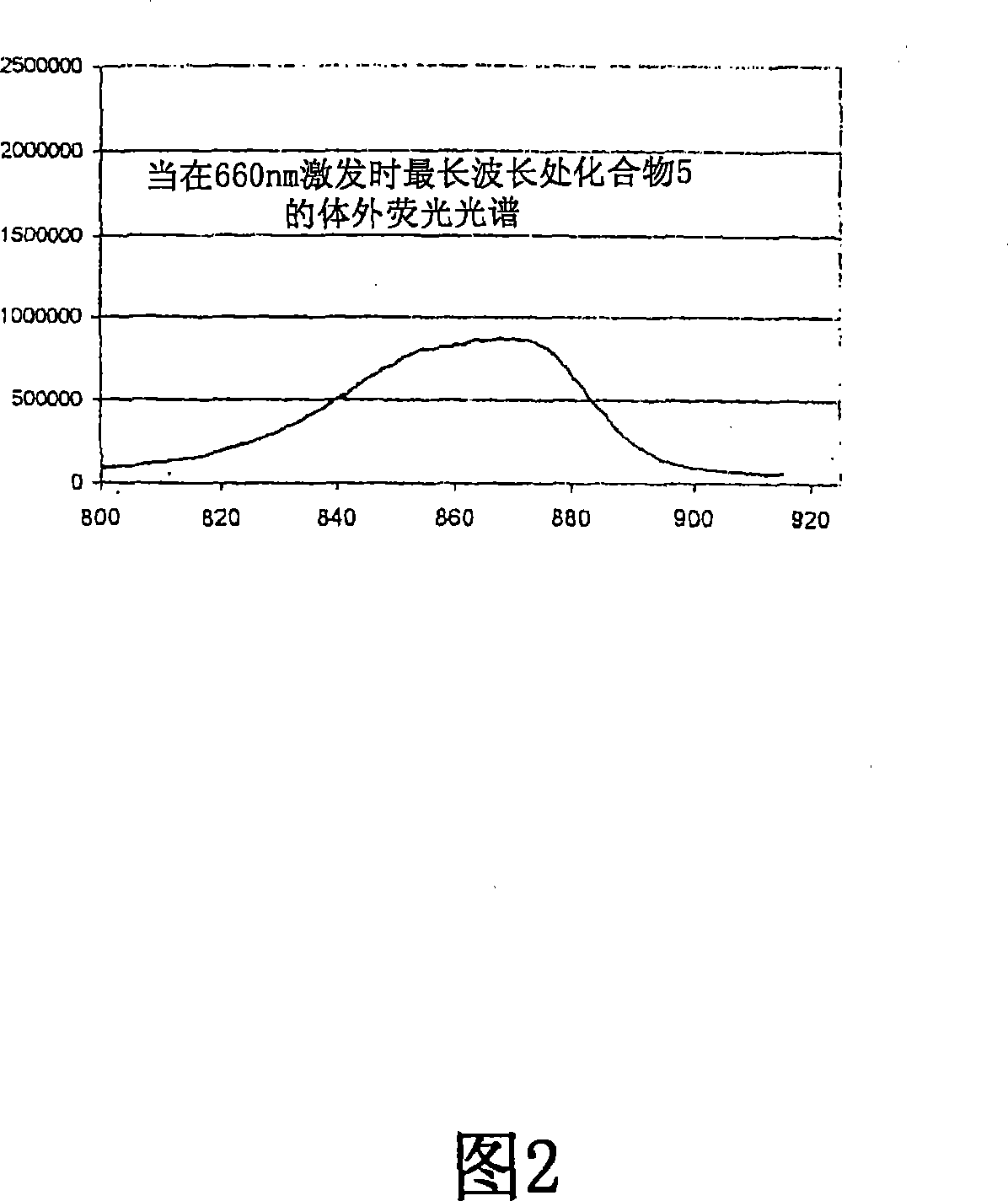
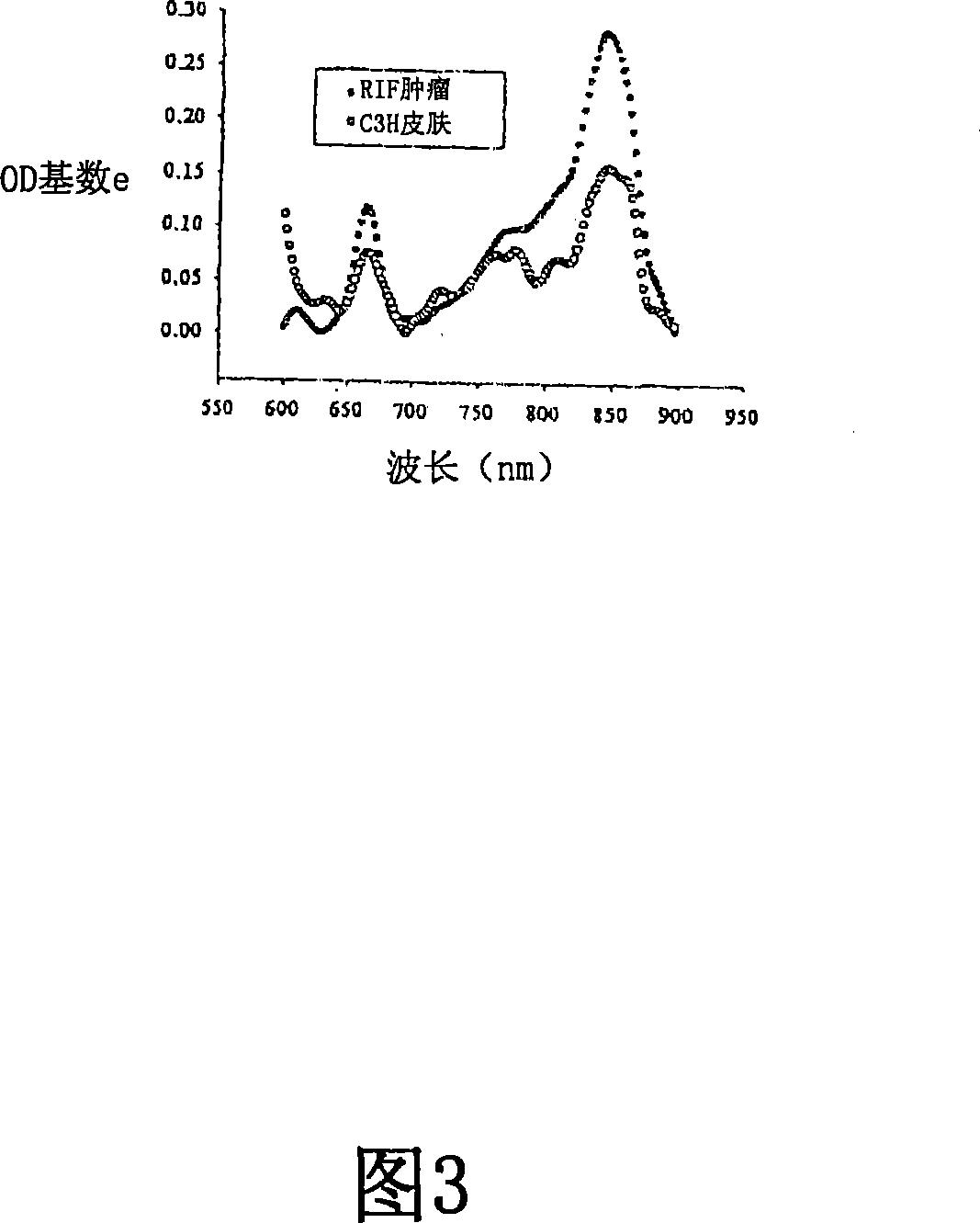
Adduct of fluorescent dye and tumor avid tetrapyrrole
一种荧光染料、染料的技术,应用在荧光染料和肿瘤亲和的四吡咯的加合物领域,能够解决缺少优选肿瘤吸收度、毒性、破坏肿瘤增生性组织等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
[0047] Specific examples of dyes used in the present invention are as follows: indocyanine green (bisindole, i.e. tricarbocyanine type dye); indocyanine green 820nm analog CAS172616-80-7 (R 7d yes ); Fast Green FCF (FD & C Green 3, a triphenylmethane dye); Supan blue (triphenylmethane dye) and methylene blue (thiazine dye).
[0048] The tumor-affinitive tetrapyrrole compound is preferably a porphyrin derivative (including porphyrin-related compounds, whether actually derived from porphyrin or not), usually selected from chlorins, bacteriochloroins and bacteriohinoids. Preferred porphyrin derivatives generally have the following general structure:
[0049]
[0050] in:
[0051] R 1 is substituted or unsubstituted -CH=CH 2 , -CHO, COOH, or
[0052] where R 9 =-OR 10 , where R 10 is a lower alkyl group of 1 to 8 carbon atoms, or -(CH 2 -O)nCH 3 ; 2 , R 2a , R3 , R 3a , R 4 , R 5 , R 5a , R 7 , and R 7a independently hydrogen, lower alkyl, substituted lowe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com