New alpha-glucosidase inhibitors from a natural source
A kind of technology of glucosidase, inhibitor, applied in the field of new natural source glucosidase inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
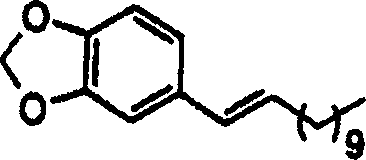
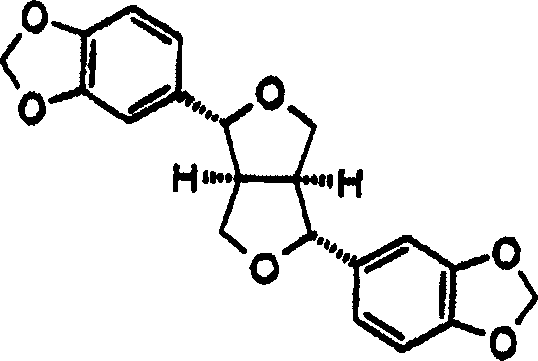
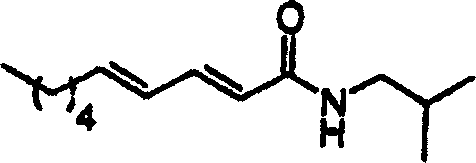
[0035] According to the purpose of the present invention, the present invention provides the method for inhibiting the α-glucosidase of individual, described method is to the individual administration effective dose pharmaceutical composition, and described composition comprises and is selected from pipataline (formula 1a), sesamin Alpha-glucosidase inhibitors of (formula 1b), parittomide (formula 1c), guineensine (formula 1d) and brachystamide-B (formula 1e) and pharmaceutically acceptable ingredients, the pharmaceutical composition can be used for the treatment of individual Suffering from diseases such as hyperglycemia, hyperinsulinemia, hyperlipoproteinemia, cancer, viral infection, hepatitis B and C, HIV and AIDS.
[0036] In one embodiment of the invention, pipataline provides α-glucosidase inhibitory activity up to 77.45%, IC 50 The value was 26.52 (μg / ml).
[0037] In yet another embodiment of the present invention, sesamin provides α-glucosidase inhibitory activity u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com