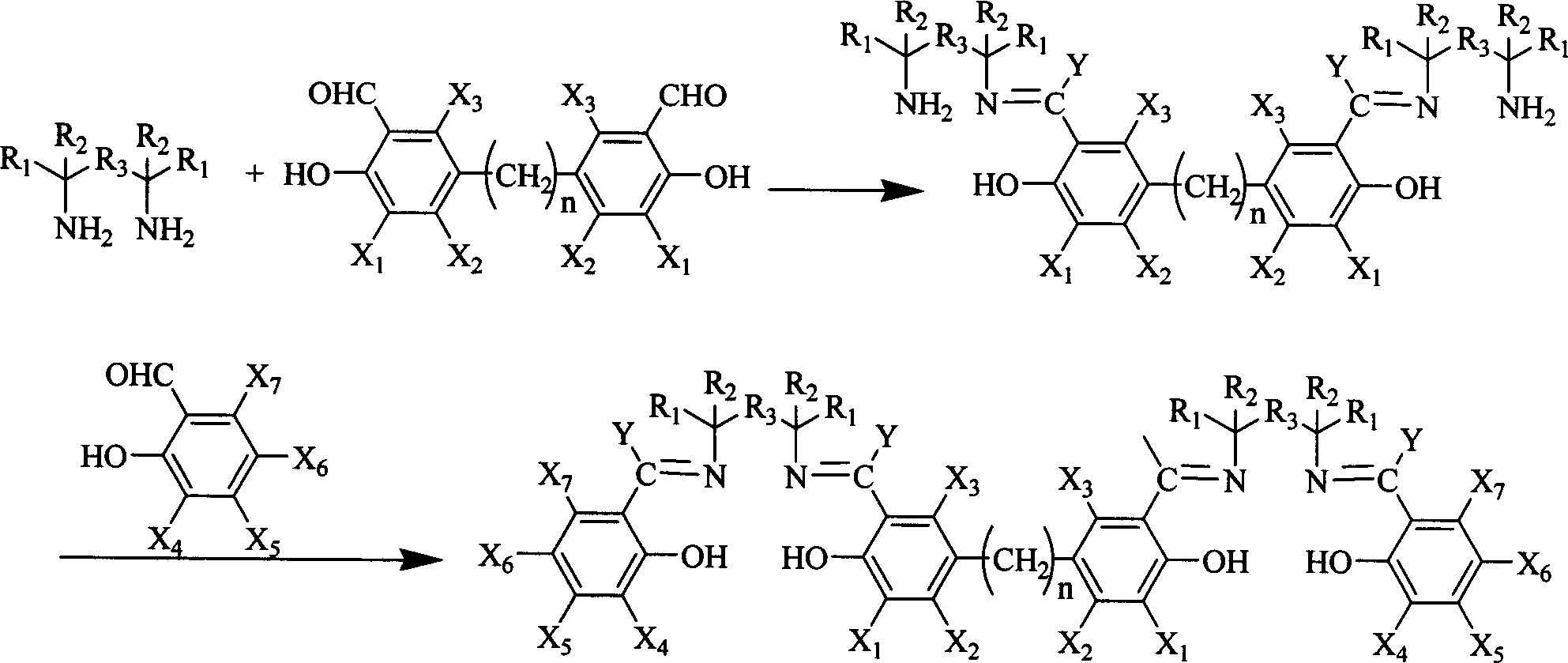
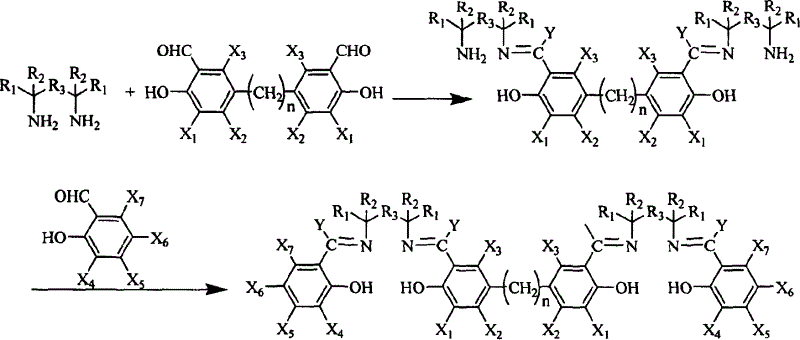
Synthesis method for preparing Salen ligand in chiral or not chiral binuclear
A synthetic method and achiral technology, applied in the field of binuclear Salen ligands, can solve problems such as troublesome product purification, and achieve the effect of reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add (R, R)-1,2-diaminocyclohexane mono(+) tartrate 2.77g in a single-necked flask of 50ml, anhydrous K 2 CO 3 2.9g, under the protection of nitrogen, add 15ml of distilled water, 6ml of ethanol, reflux for 3h. with 30mlCH 2 Cl 2 The released cyclohexanediamine, CH 2 Cl 2 layer anhydrous Na 2 SO 4 dry. Dried CH 2 Cl 2 layer to filter out Na 2 SO 4 After that, it was added to a 100ml single-necked flask, cooled to 0°C in an ice bath, and 23ml CH 2 Cl 2 The solution was dripped in 1 hour, and kept in an ice bath for 24 hours. Then, add dropwise 1.74 g of 5,5′-methylene bis-tert-butyl salicylaldehyde in 17 ml of CH 2 Cl 2 solution. After stirring the reaction at room temperature for 12 h, the reaction was detected by TLC, and there was no product point.
Embodiment 2
[0017] The conditions are the same as in Example 1, except that the order of addition of 3,5-di-tert-butyl salicylaldehyde and 5,5'-methylene bis-tert-butyl salicylaldehyde is changed, that is, 5,5'-methylene bis After tert-butyl salicylaldehyde was reacted for 24 hours, 3,5-di-tert-butyl salicylaldehyde was added. After the reaction was completed, TLC detection showed only a small amount of product.
[0018] As can be seen from the above two examples, the method for extracting cyclohexanediamine is not easy to obtain dinuclear ligands. Therefore, we adopt the method of not extracting cyclohexanediamine, that is, directly add 3,5-di-tert-butyl salicylaldehyde or 5,5'-methylene bis-tert-butyl after cyclohexanediamine is freed from its salt For the salicylaldehyde reaction, the following examples are the one-pot method for synthesizing the dinuclear Salen ligand without extracting cyclohexanediamine.
Embodiment 3
[0020] Add (R, R)-1,2-diaminocyclohexane mono(+) tartrate 1.901g in a 100ml single-necked flask, anhydrous K 2 CO 3 2.0g, under the protection of nitrogen, add 6ml of distilled water and stir at room temperature for 10min to dissolve the solid. Add 50ml CH 2 Cl 2 , add 12g K in two times 2 CO 3 Absorbs water inside. Cool down to 0°C in an ice bath, and start to add dropwise 5,5'-methylenebis-tert-butylsalicylaldehyde 1.104g in 20ml of CH 2 Cl 2 The solution was dripped in 1 hour, and kept in an ice bath for 24 hours. Then, add dropwise 2.527 g of 3,5-di-tert-butyl salicylaldehyde in 10 ml CH 2 Cl 2 solution. After the dropwise addition, the solid was filtered, and 80 ml of absolute ethanol was added to the solution. Reflux for 9 hours, TLC detects the reaction, and the chromatographic yield of the product is 70%. First, 80ml of solvent was evaporated, and a solid precipitated after standing still, which may be a mixture of trinuclear and dinuclear catalyst ligands....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com