Thienodibenzoazulene compounds as tumor necrosis factor inhibitors
A technology of compounds and solvates, applied in the field of 1-thiadibenzoazulene derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0068] Preparation of Alcohol
[0069] To LiAlH 4 A suspension in anhydrous diethyl ether (10mmol / 15ml anhydrous diethyl ether) was added dropwise to a solution of ester in diethyl ether (2mmol / 15ml anhydrous diethyl ether). The reaction mixture was stirred at room temperature for 4 hours. Subsequently, when all esters were consumed in the reaction (reaction progress monitored by TLC), diethyl ether and water were added to decompose the excess LiAlH 4 . The resulting white precipitate was filtered off and washed with anhydrous Na 2 SO 4 After drying, the filtrate was evaporated under reduced pressure. The crude product was purified by column chromatography.
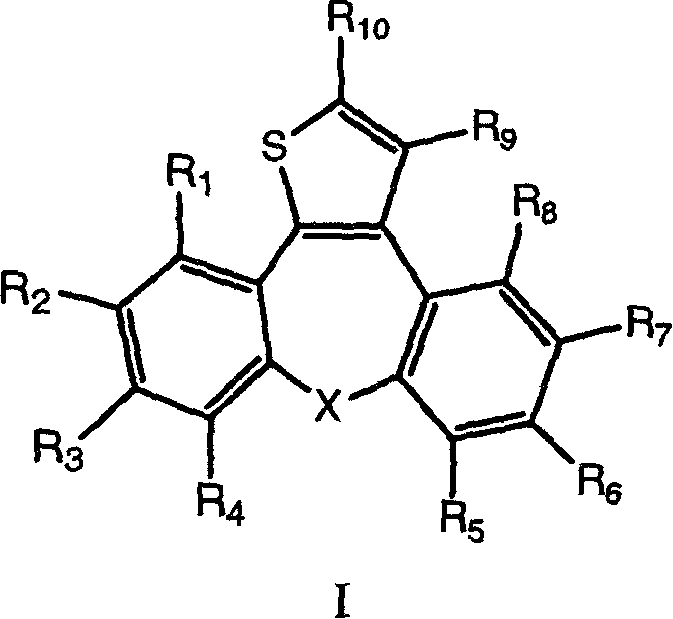
[0070] According to the method of Example A, starting from the corresponding ester, prepare dibenzoazulene alcohol represented by formula I, wherein R 1 , R 5 , R 6 , R 7 , R 8 and R 9 = H, R 2 , R 3 , R 4 and X have the meanings described in Table 1.
[0071] compound
Embodiment 1
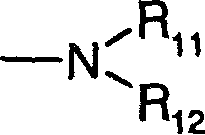
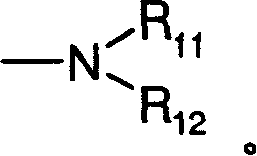
[0074] Dimethyl-[3-(8-oxa-1-thia-dibenzo[e,h]azulene-2-ylmethoxy)-propyl]-amine hydrochloride
[0075] Benzyltriethylammonium chloride (0.1 g, 0.44 mmol) and alcohol 1 (0.28 g , 0.001mol) toluene solution. The reaction mixture was heated to reflux for 4 hours with vigorous stirring. It was then cooled to room temperature, diluted with water and extracted with dichloromethane. After purification by column chromatography, the oily product (0.25 g) was isolated. Concentrated hydrochloric acid was added to a cold ethanol solution of the amine to give a crystalline product, m.p. 162-165°C.
[0076] C, H, N, S analysis: C 65.45 (calculated 65.74); H 6.12 (calculated 6.02); N 3.89 (calculated 3.48); S8.52 (calculated 7.98)
[0077] 1 H NMR (ppm, CDCl 3 ): 2.18(m, 2H); 2.79(d, 6H); 3.15(m, 2H); 3.68(t, 2H); 4.71(s, 2H); 1H).
Embodiment 2
[0079] Dimethyl-[2-(8-oxa-1-thia-dibenzo[e,h]azulene-2-ylmethoxy)-ethyl]-amine hydrochloride
[0080] Reaction of alcohol 1 (0.45 g, 0.0015 mol) with 2-dimethylaminoethyl chloride hydrochloride (3.05 g, 0.021 mol) gave an oily product (0.3 g), converted to hydrochloride, m.p. 203°C .
[0081] C, H, N analysis: C 64.85 (calculated 65.02); H 5.80 (calculated 5.72); N 3.48 (calculated 3.61).
[0082] 1 H NMR (ppm, CDCl 3 ): 2.89(s, 6H); 3.27(m, 2H); 4.07(m, 2H); 4.78(s, 2H); 7.16-7.47(m, 9H); 12.5(s, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com