Compositions containing endocannabinoid mimetics and anti-inflammatory compounds, methods of preparation and uses thereof
A technology for endocannabinoids and analog compounds, which is applied in the directions of medical preparations containing active ingredients, drug combinations, active ingredients of hydroxyl compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
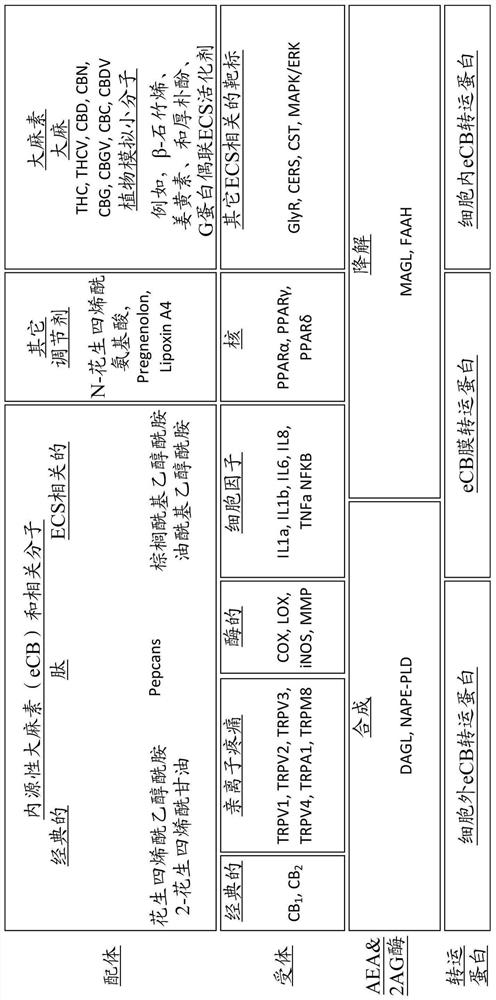
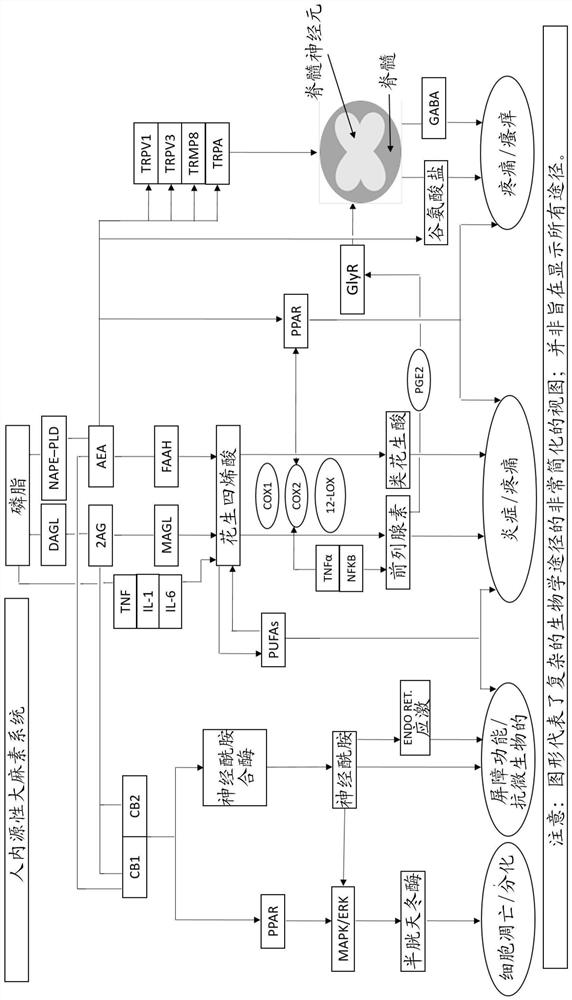
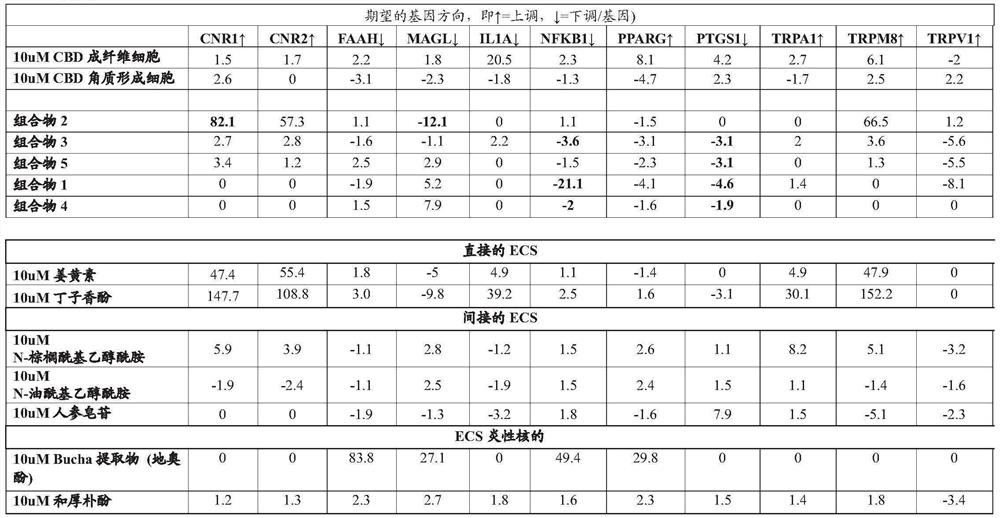
[0218] Some compositions of the invention comprising ECS direct, indirect, related inflammatory pathway and TRP related pathway compounds regulate multiple genes (eg CB1, CB2, FAAH, MAGL, IL1a) significantly better than cannabidiol in fibroblasts , NFKB, PRGS1, TRPM8 and TRPV1) beneficial gene expression (e.g. 54-fold better for CNR1, 33-fold better for CNR2, 15-fold better for MAGL, 10-fold better for TRPM8), indicating that the plants of the invention mimic non-cannabis Cannabinoid compounds favorably modulate ECS more effectively than traditional cannabinoids. See Test Example 1, Compositions 2 and 3.
[0219] In another embodiment, the composition comprises a combination of compounds designed for anti-inflammatory, anti-aging, skin matrix improvement and wound healing selected from direct and indirect endocannabinoid compounds from three Anti-inflammatory compounds for each of the ECS-related anti-inflammatory pathways (nuclear, enzymatic, and cytokines) and the ECS-rel...
Embodiment 3
[0227] Similarly, the compositions of the present invention are in keratinocytes in regulating various genes of skin matrix function, including those for lipid regulation / barrier repair (CERS3, FLG, TLR2), those for inflammatory responses (IL1a, NFKB, MMP1) and structural pathway genes affecting extracellular matrix protein metabolism and cell proliferation, differentiation and apoptosis (COL1A1, ITGB1, JUN, KLF4) regulate beneficial gene expression, superior to cannabidiol in CERS3 , COL1A1, FLG, IL1A, ITGB1, JUN & KLF4, which again demonstrate that the plant-mimetic non-cannabinoid compounds of the present invention are more potent and beneficial in modulating ECS than conventional cannabinoids. See Test Example 3, Composition 8.
[0228]In one embodiment, the composition comprises at least one direct endocannabinoid mimetic compound, wherein the at least one compound is demethoxycurcumin, bisdesmethoxycurcumin, tetrahydroturmeric fenugreek, eugenol, or any combination th...
Embodiment 2
[0424] cell culture : Human skin keratinocyte cultures (or cultures) were obtained by ThermoFisher Scientific (Waltham, MA) or PromoCell GmbH (Heidelberg, Germany). The initial culture was selected from one of the following: C055C, 3C0647 or C12003. Cell lines were derived from the following donors, as follows:
[0425] C055C: Single Adult Donor
[0426] 3C0647: Single Adult Donor, Lightly Pigmented
[0427] C12003: Single Adult Donor
[0428] culture medium : Cells were ready for culture using keratinocyte growth medium from PromoCell GmbH (Heidelberg, Germany) supplemented with bovine pituitary extract 0.004ml / ml, epidermal growth factor (recombinant human) 0.125ng / ml , insulin (recombinant human) 5μg / ml, hydrocortisone 0.33μg / ml, epinephrine 0.39μg / ml, transferrin (recombinant human) 10μg / ml and CaCl 2 0.06mM basal medium. During the 24 hour experimental period, cells were maintained only in basal medium supplemented with test compounds. Store all cultures at 37 °...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com