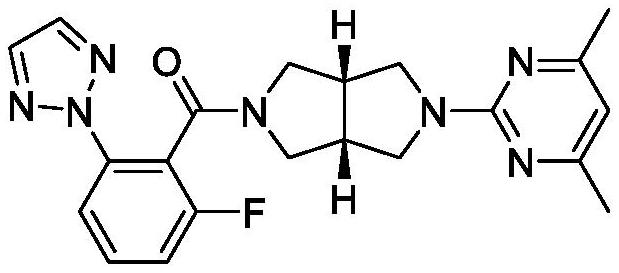
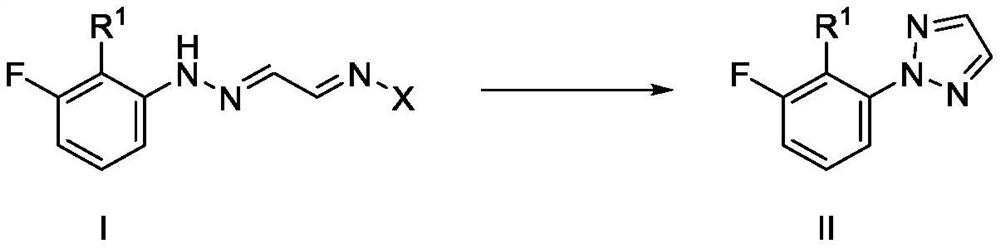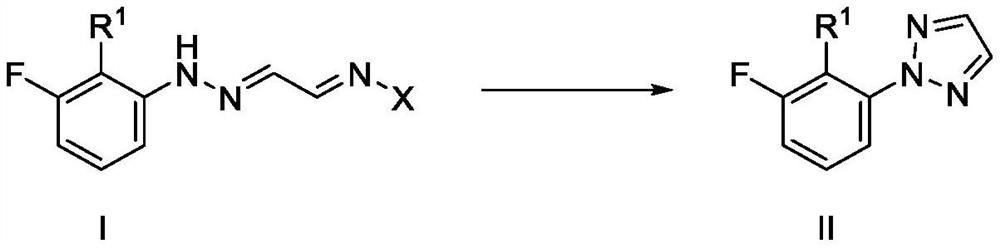Improved synthetic process for preparing (2H-1, 2, 3-triazol-2-yl) phenyl compounds as orexin receptor modulators
A -2H-1, phenyl technology, applied in the preparation of hydrazone, drug combination, organic chemistry and other directions, can solve problems such as low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Example 1: Synthesis of hydrazide acetaldehyde of formula IV
Embodiment 1a
[0142] Example 1a: Synthesis of (E)-2-(2-(3-fluorophenyl)hydrazono)acetaldehyde.
[0143]
[0144] A 40 w / w% solution of glyoxal in water (613 g, 4.22 mol) was added to a suspension of 177 g (1.06 mol) (3-fluorophenyl)hydrazine (hydrochloride) in 1.24 L of water, followed by 2 hours A solution of 129.9 g (1.58 mol) of sodium acetate in 708 mL of water was added. After stirring at room temperature for several hours, the suspension was filtered and the filter cake was washed with 0.89 L of water and dried in vacuo to afford 172.8 g (95% yield) of the title compound as a yellow solid.
[0145] mp 118°C-119°C.
[0146] 1 H NMR (DMSO-d 6)δ: 11.80(br s, 1H), 9.49(d, J=7.7Hz, 1H), 7.36(d, J=7.9Hz, 1H), 7.32-7.39(m, 1H), 6.96-7.03(m, 2H), 6.75-6.83 (m, 1H). 13 C NMR (DMSO-d 6 )δ: 190.4, 163.0 (br d, J=242.7Hz), 144.7 (br d, J=10.8Hz), 136.3, 131.2 (d, J=10.0Hz), 110.0 (d, J=2.3Hz), 108.5 (d, J=21.6 Hz), 100.6 (d, J=27.0 Hz). 19 F NMR (DMSO-d 6 )δ: -111.72.
[0147] HRMS...
Embodiment 1b
[0148] Example 1b: Synthesis of (E)-methyl 2-fluoro-6-(2-(2-oxoethylidene)hydrazino)benzoate
[0149]
[0150] A solution of methyl 2-fluoro-6-hydrazinobenzoate (17.65g, 0.08mol) in methanol-water (90ml+180ml) was added to 40w% aqueous glyoxal ( 58.04g, 0.8mol), water (100ml) and sodium acetate (9.85g, 0.12mol). The mixture was then stirred for about 1.5 hours and then filtered. The filter cake was washed with water (2 x 50 mL) and dried in vacuo. The dry solid (16.12g) was redissolved in ethyl acetate (50ml) at 50°C, then crystallized by slow addition of heptane (200ml) and cooling to 5°C. The resulting solid was filtered, rinsed with heptane (2 x 15ml) and dried in vacuo. The desired product was obtained as a yellow solid (13.33 g, 74% yield). mp 110.8°C.
[0151] 1 H NMR (DMSO-d 6 )δ: 11.74(s, 1H), 9.41(d, 1H), 7.49(m, 2H), 7.19(d, 1H), 6.92(m, 1H), 3.84(s, 3H).
[0152] MS(ESI-TOF) m / z: 225.1 ([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com