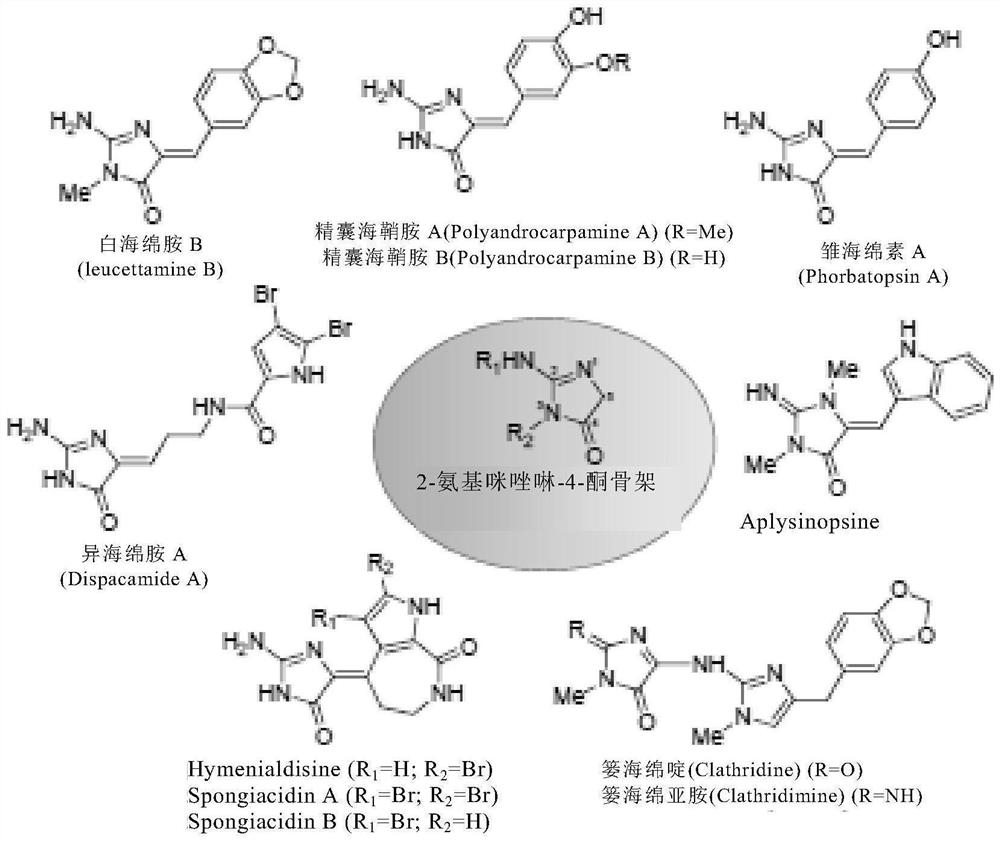
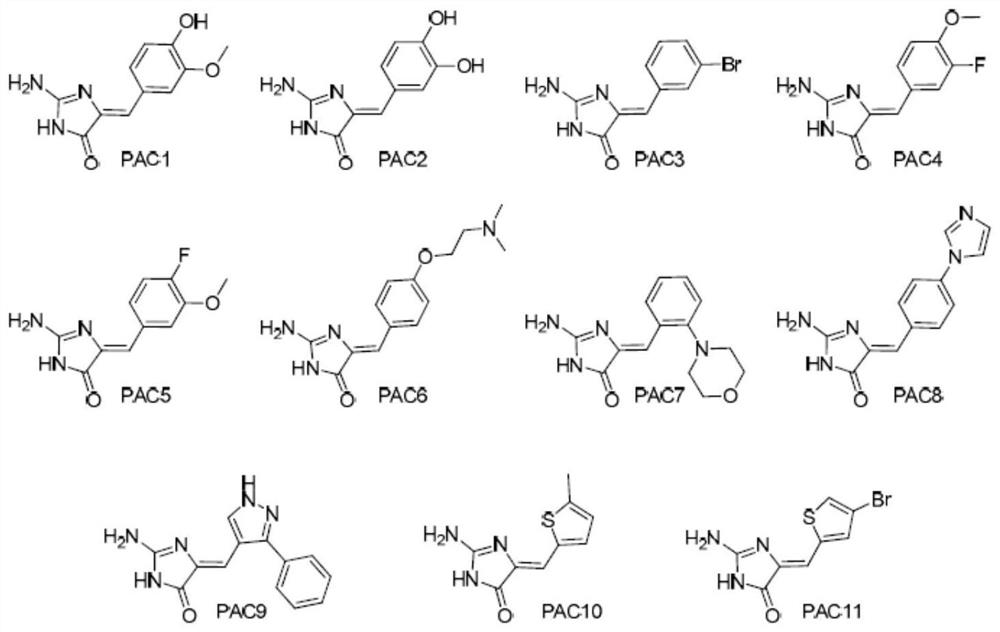
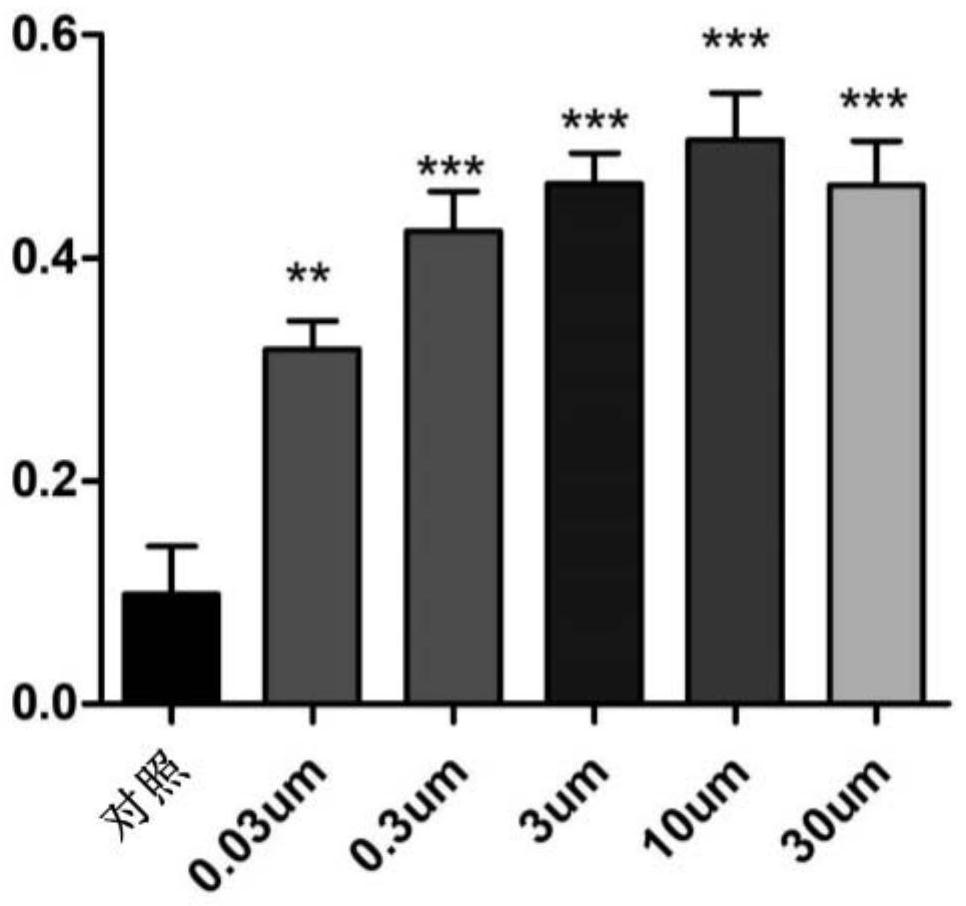
Use of heterocyclic derivatives having cardiomyocyte proliferative activity for treatment of heart diseases
A technology for cardiomyocytes and uses, applied in the field of medical technology and medicine, can solve the problem of not being able to replace cardiomyocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0117] The present invention provides processes for the preparation of compounds of formula (I). The compounds of the present invention can be readily prepared by various synthetic procedures familiar to those skilled in the art. Exemplary preparations of these compounds may include, but are not limited to, the procedures described below.
[0118] Generally, in the preparation process, each reaction is generally carried out in an inert solvent at room temperature to reflux temperature (eg, 0-150°C, preferably 0-100°C). The reaction time is usually 0.1 to 60 hours, preferably 0.5 to 48 hours.
[0119] Preferably, the compounds of formula (I) of the present invention can be prepared with reference to the following schemes. In practice, the steps of the method can be extended or combined as desired.
[0120] plan 1
[0121]
[0122] The scheme shows one route that can be used to obtain the targets synthesized in this patent. Intermediate I-1 can be used as described in Ro...
Embodiment 1
[0149] Example 1: (Z)-5-(benzo[d][1,3]dioxol-5-ylmethylene)-2-(methyl(phenyl)amino)-3, 5-Dihydro-4H-imidazol-4-one
[0150]
[0151] To a solution of benzo[d][1,3]dioxol-5-ylmethanol (10.0 g, 65.7 mmol) in DCM (150 mL) was added activated MnO 2 (57.0 g, 657 mmol) and the mixture was stirred at 40 °C for 17 h. After filtration, the filtrate was concentrated to give benzo[d][1,3]dioxol-5-carbaldehyde (9.8 g, 99%) as a white solid, which was used in the next step without further purification. LRMS(M+H + ) m / z calculated value 151.0, measured value 151.0.
[0152]
[0153] To 2-thioimidazolidin-4-one (1.9 g, 17 mmol) and benzo[d][1,3]dioxol-5-carbaldehyde (3.0 g, 20.4 mmol) in toluene (30 mL) Piperidine (71 mg, 0.85 mmol) was added to the mixture in , and the mixture was stirred at 120 °C for 19 h. After concentration, the residue was recrystallized from DCM (30 mL) to give (Z)-5-(benzo[d][1,3]dioxol-5-ylmethylene)-2- Thioimidazolidin-4-one (3.8 g, 90%) as a yellow sol...
Embodiment 2
[0156] Example 2: (Z)-5-(benzo[d][1,3]dioxol-5-ylmethylene)-3-benzyl-2-(benzyl(phenyl) amino)-3,5-dihydro-4H-imidazol-4-one
[0157]
[0158]
[0159] To (Z)-5-(benzo[d][1,3]dioxol-5-ylmethylene)-2-(phenylamino)-3,5-diol at room temperature To a mixture of hydrogen-4H-imidazol-4-one (46 mg, 0.15 mmol), benzyl alcohol (21 mg, 0.195 mmol) and triphenylphosphine (59 mg, 0.225 mmol) in THF (1 mL) was added DIAD (46 mg, 0.225 mmol) mmol). in N 2Under protection, the mixture was stirred at room temperature for 21 h. The reaction mixture was concentrated and the residue was purified by silica gel flash chromatography (EA / PE=1 / 1, v / v) to give (Z)-5-(benzo[d][1,3]dioxane Penten-5-ylmethylene)-3-benzyl-2-(benzyl(phenyl)amino)-3,5-dihydro-4H-imidazol-4-one (10 mg, 9%) as Yellow solid.
[0160] LRMS(M+H + ) m / z calculated value 488.2, measured value 488.2. 1 H NMR(400MHz,DMSO-d6)δ7.29-6.95(m,16H),6.60(t,J=20.0Hz,3H),6.08(s,2H),4.79(s,2H),4.34(s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com