MMLV enzyme combination mutant
A technology of mutants and site mutations, applied in the field of combined mutants of MMLV enzymes, can solve problems such as uncertainty in enzyme transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
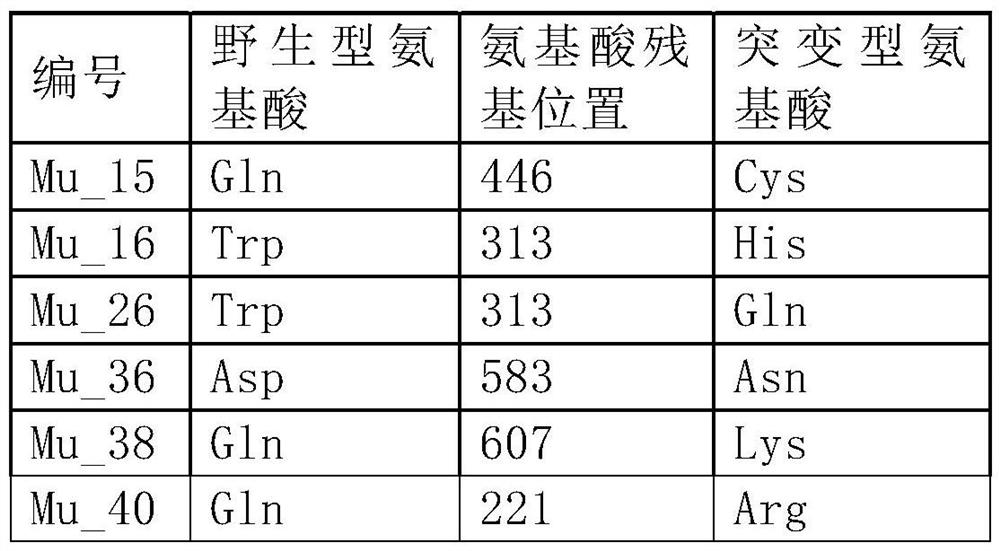
[0120] Embodiment 1 Calculation and screening of DDG value of each point
[0121] The MMLV protein sequence was input into the Rosetta algorithm software Cyrus Bench (Cyrus Biotechnology), and the 0-100, 101-200, 201-300, 301-400, 401-500, 501-600, and 601-671 amino acids were segmented for all positions. The DDG value of the point full mutation is calculated, and the information of the mutation site with a significantly reduced DDG value (DDG value <-2) is obtained as follows:
[0122] Table 1
[0123]
[0124]
Embodiment 2
[0125] Example 2 Construction of MMLV mutation library
[0126] According to the above protein sequence, codon optimization was performed by Suzhou Jinweizhi Biotechnology Co., Ltd., and the DNA sequence (SEQ ID NO.: 2) was compiled.
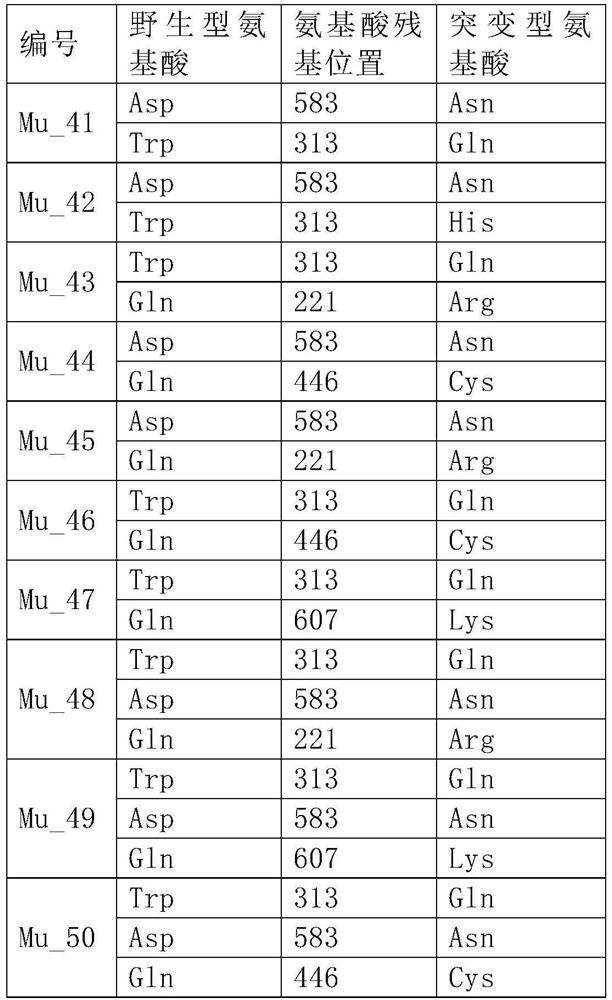
[0127] Gene synthesis was carried out by Suzhou Jinweizhi Biotechnology Co., Ltd. according to the above DNA sequence, 5'(NheI) and 3'(XhoI) restriction enzyme sites were added, and the gene was cloned into the vector pET28a through 5'NheI and 3'XhoI, The plasmid WT-pET28a was constructed, the recombinant plasmid DNA and the glycerol bacteria containing the recombinant plasmid were prepared, and the plasmid WT-pET28a was site-directed mutagenesis according to the mutation sites involved in Example 1 to construct a mutation library Mu1-pET28a~Mu40-pET28a .
Embodiment 3
[0128] Example 3 Expression and purification of MMLV mutants
[0129] The WT-pET28a, Mu1~40-pET28a plasmids were transformed into BL21(DE3) competent cells to obtain 37 expression host bacteria, and then transferred to 3ml LB medium, shaken at 37°C for 5 hours, and then added 0.1Mm IPTG at 18°C Induction culture overnight. The induced cells were collected, lysing solution (50Mm Tris, 50Mm NaCl, pH7.5) was added, lysed by ultrasonic, and the supernatant was separated by centrifugation. The supernatant was taken and purified by Ni NTA metal ion chelating packing to obtain wild-type and 40 mutant MMLV proteins
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com