Personalized treatment of ophthalmic diseases
A disease-based, diabetic technology for the personalized treatment of ophthalmic diseases that addresses instability, incomplete resolution, and heavy burden on patients and healthcare providers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0663] Treatment of patients with vascular eye diseases using bispecific antibodies that bind to human VEGF and to human ANG2
example 1
[0665] Efficacy and durability of treatment in patients with neovascular age-related macular degeneration (nAMD) using individualized treatment intervals
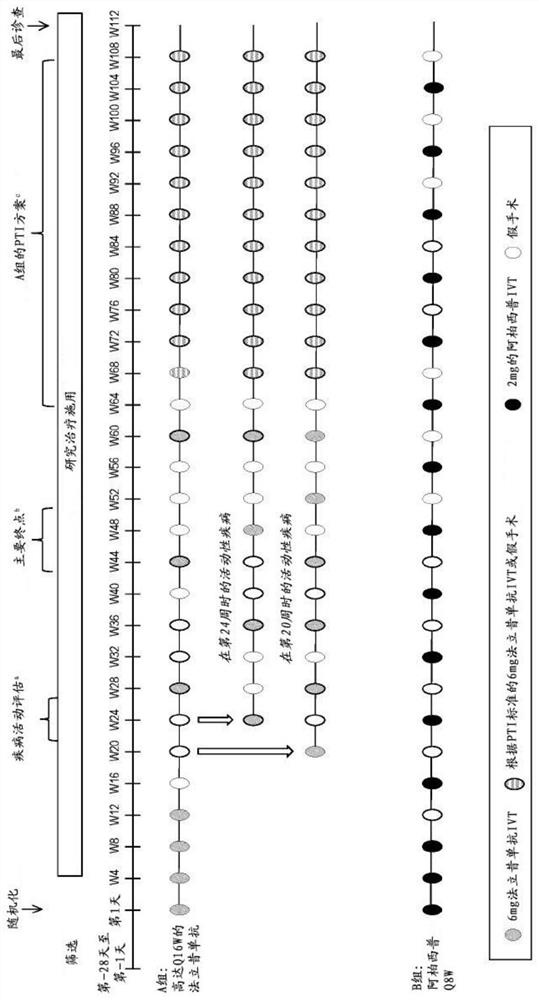
[0666] In an earlier 52-week phase II investigative study, it was seen, inter alia, that in all patients involved, RO6867461 (farciximab) was effective every 12 weeks in treatment-naïve nAMD patients. The efficacy of weekly and 16 week administration was longer lasting (longer time required for retreatment). Three arms were studied - arm A (Q12W): 6 mg RO6867461 intravitreal (IVT) every 4 weeks to week 12 (4 injections), then 6 mg RO6867461 IVT every 12 weeks to week 48 (at weeks 24, 36 and 48 Injection; 3 injections) ……………………………—Arm B (Q16W): 6 mg RO6867461 IVT every 4 weeks to Week 12 (4 injections), then 6 mg RO6867461 IVT every 16 weeks to Week 48 Weeks (injections at Weeks 28 and 44; 2 injections)……………………………………………—Group C (comparison group): 0.5 mg ranibizumab IVT every 4 weeks to At week 48 (13 injections), only...
example 2
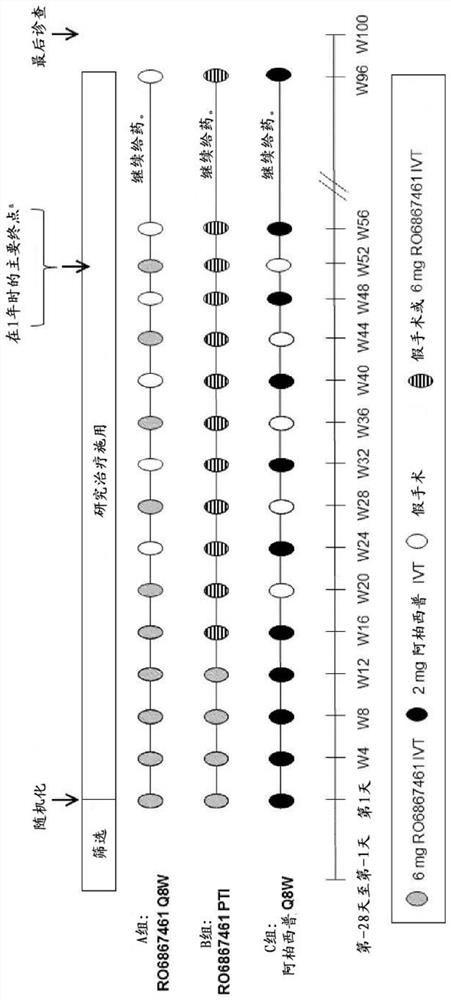
[0745] Efficacy and durability of bispecific anti-VEGF / ANG2 therapy in patients with diabetic macular edema (DME) using individualized treatment intervals
[0746] In an earlier phase II study of 36 weeks in patients with diabetic macular edema (DME), some degree of potential longer persistence (possibly longer retreatment time) was seen in all relevant patients ). The three study groups were treated as follows: Group A: 0.3 mg ranibizumab, intravitreal (IVT); Group B: 1.5 mg RO6867461 (Fariximab), IVT; monoclonal antibody), IVT.
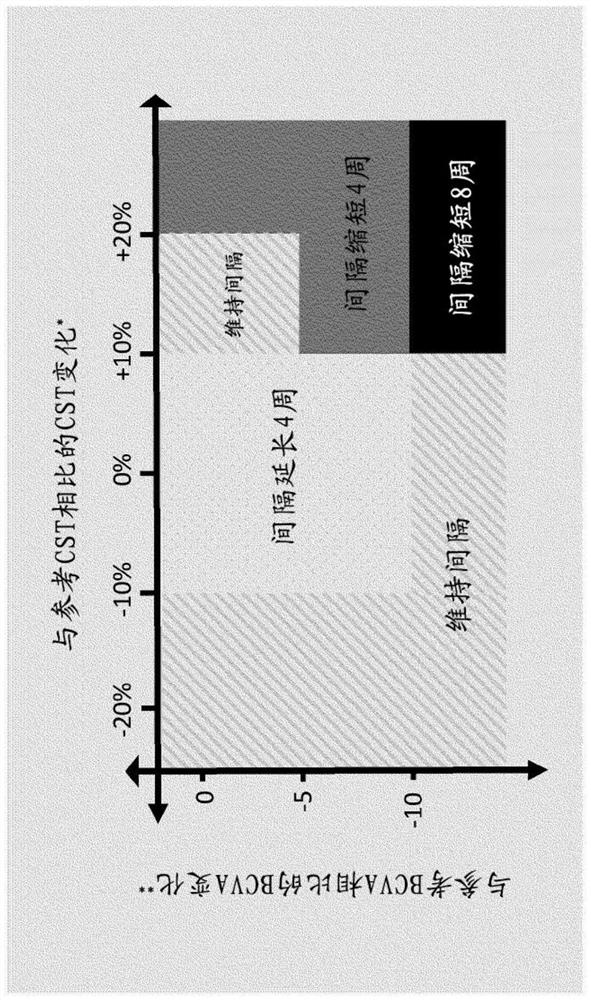
[0747] Results regarding potential longer retreatment times for RO6867461 (fariximab, VA2) Figure 6 shown in . Figure 6 Time to retreatment in DME patients after cessation of dosing (after 20 weeks or 6 monthly doses = time after last intravitreal (IVT) administration) is shown, based on disease activity as assessed by: BCVA reduction ≥5 letters, and a CST increase of ≥50 μm (=patients with an event). Bispecific anti-VEGF / ANG2 antibody...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com