Method and device for identifying adverse drug reaction
A technology of adverse reactions and identification methods, applied in drug reference, patient-specific data, structured data retrieval, etc., can solve problems such as missed reports, reduce accurate judgment of adverse reactions of drugs, and cannot clarify the number of "denominators", achieving good results External authenticity, short time-consuming, time-consuming and economical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
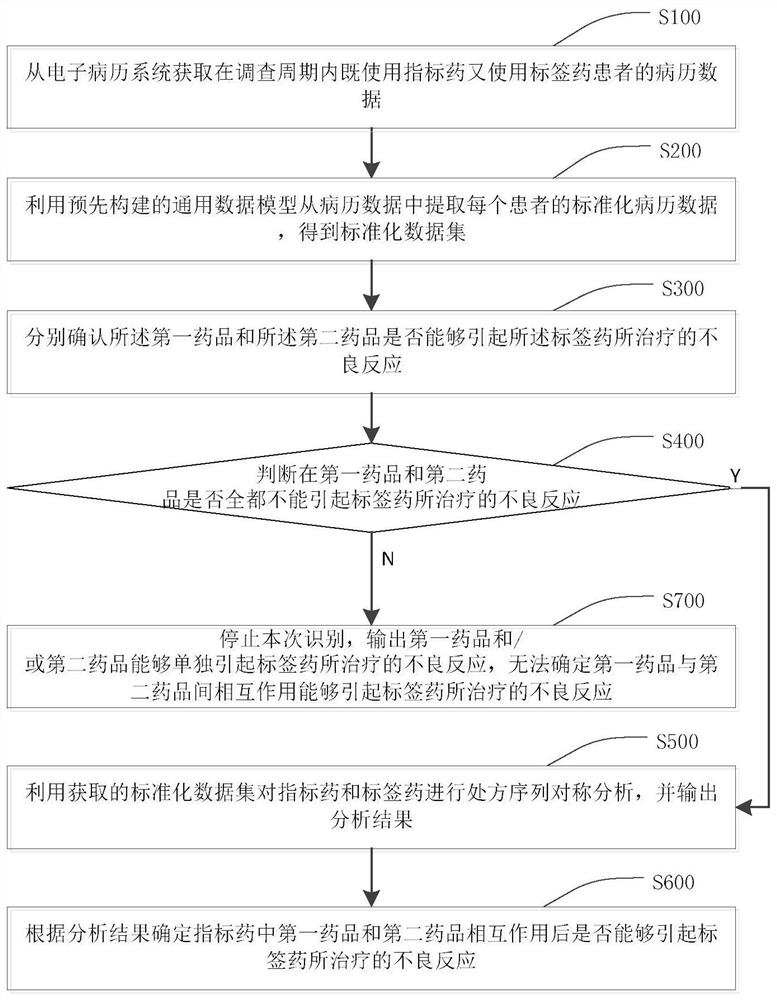
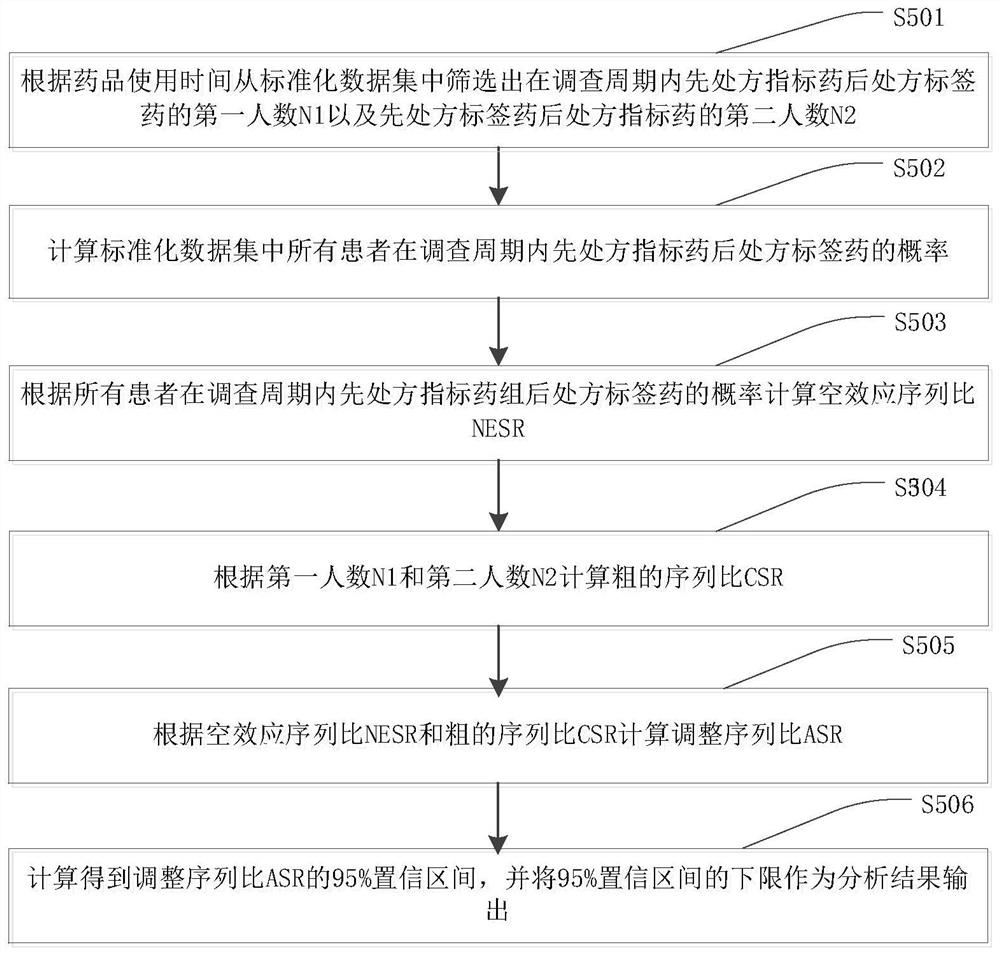
[0056]In order to make the purpose, technical solutions and advantages of this application clearer, the following in conjunction with the attached Figure 1-4 And embodiment, this application is described in further detail. It should be understood that the specific embodiments described here are only used to explain the present application, not to limit the present application.
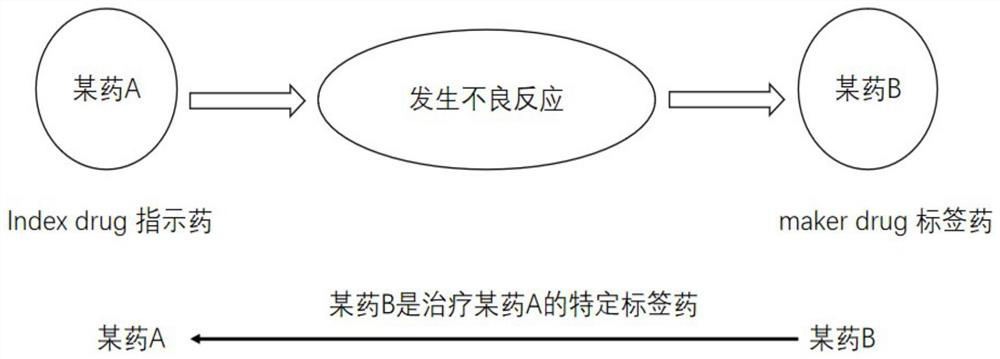
[0057] Such as figure 1 As shown, the prescription symmetry analysis is based on the order and frequency distribution of drug use to determine whether there is a relationship between the drug and the adverse reaction event; the prescription symmetry analysis can use the label drug B to indicate the index drug A, and find that a certain drug A causes the adverse reaction events of the label drug B. reaction. This application further expands the prescription symmetry analysis. Through the interaction between the index drug A and the index drug C through the label drug B, it is found that the drug A an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com