Pharmaceutical application of beta-catenin specific inhibitor
A catenin, specific technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
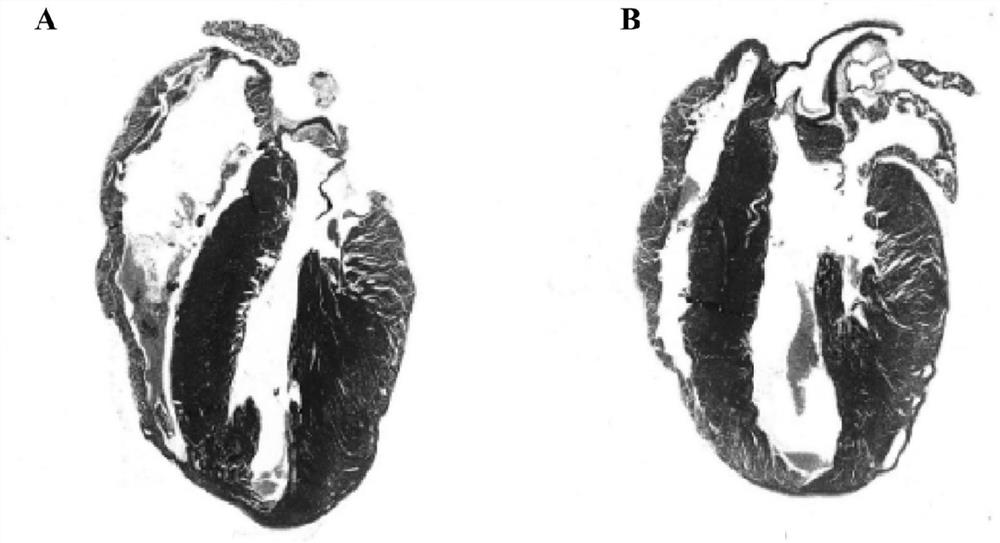
[0022] Example 1 Comparison of heart size in mice with dilated cardiomyopathy before and after administration
[0023] This experiment is a comparison experiment of heart size before and after administration of cardiomyopathy mice. figure 1 A mouse model of right ventricle-dominant dilated cardiomyopathy caused by DSC2 deletion. The size of the mouse heart was detected by echocardiography, and the mice were given drug intervention when the cardiac cavity of the mouse became larger. figure 1 A represents cardiomyopathy mice-placebo group; figure 1 B shows the β-catenin-IN-2 administration group of cardiomyopathy mice. Through multiple experiments, it was found that the mice with dilated cardiomyopathy showed significantly enlarged hearts after intraperitoneal injection of placebo in the control group ( figure 1 A), while the β-catenin-IN-2 administration group did not change the heart ( figure 1 B).
Embodiment 2
[0024] Example 2 Comparison of myocardial apoptosis before and after administration of cardiomyopathy mice
[0025] This experiment is a comparison of myocardial apoptosis before and after administration of cardiomyopathy mice. figure 2 A mouse model of right ventricle-dominant dilated cardiomyopathy caused by DSC2 deletion. The apoptosis-related detection of mouse cardiomyocytes was performed by Tunel, and the mice were subjected to drug intervention when the cardiac cavity of the mice became larger. in figure 2 A (WT group), figure 2 B (WT+IN-2 group), figure 2 C (KO group), figure 2 The D(KO+IN-2) group represents the normal control mice-placebo group, the normal control mice-administration group, the cardiomyopathy mice-placebo group, and the cardiomyopathy mice-administration group, respectively. Through multiple experiments, it was found that the cardiomyopathy mice showed a significant increase in myocardial apoptosis after intraperitoneal injection of placebo...
Embodiment 3
[0026] Example 3 Comparison of myocardial contractility before and after administration of cardiomyopathy mice
[0027] This experiment is a comparison of myocardial contractility before and after administration of cardiomyopathy mice. image 3 The contractility of cardiomyocytes in mice with dilated cardiomyopathy caused by DSC2 deletion. image 3 A, 3B, and 3C represent the cell length, maximal systolic rate, and maximal diastolic rate when a single cardiomyocyte contracts; and in each figure, the WT group, KO group, and KO+IN-2 group represent normal mice-placebo group, Cardiomyopathy mice-placebo group and cardiomyopathy mice-administration group. Through the single-contraction experiment of isolated KO mouse cardiomyocytes, it was found that after intraperitoneal injection of placebo, cardiomyocytes in cardiomyopathy mice showed a decrease in cardiomyocyte contractility, but in cardiomyopathy mice intraperitoneal injection of β-catenin-IN- After 2, the contractility of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com