Continuous process for preparation of anticholinergic agents
A technology for anticholinergic drugs and drugs, which is applied in the continuous field of preparing anticholinergic drugs and can solve problems such as large-scale production and saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The methods for the preparation of anticholinergics / drugs disclosed herein may include the use of one or more flow programs to perform a continuous flow mode process.
[0036]The term "flow procedures" as used herein relates to procedures, for example, using certain devices and / or certain conditions required to enable chemical synthesis to run continuously. Flow procedures as used herein do not include traditional batch processes. Preferably, a continuous reactor is used to deliver the materials in a flowing stream, as will be understood by those skilled in the art.
[0037] A process may be defined as continuous in that it is characterized by the continuous feeding of reactants into the reactor while the continuous formation and withdrawal of a product stream.
[0038] The continuous process disclosed in this invention may be advantageous for a number of reasons including, but not limited to, improved purity and yield of product and reduced effluent; thus, the process...
Embodiment 1
[0063] Preparation of umeclidinium bromide
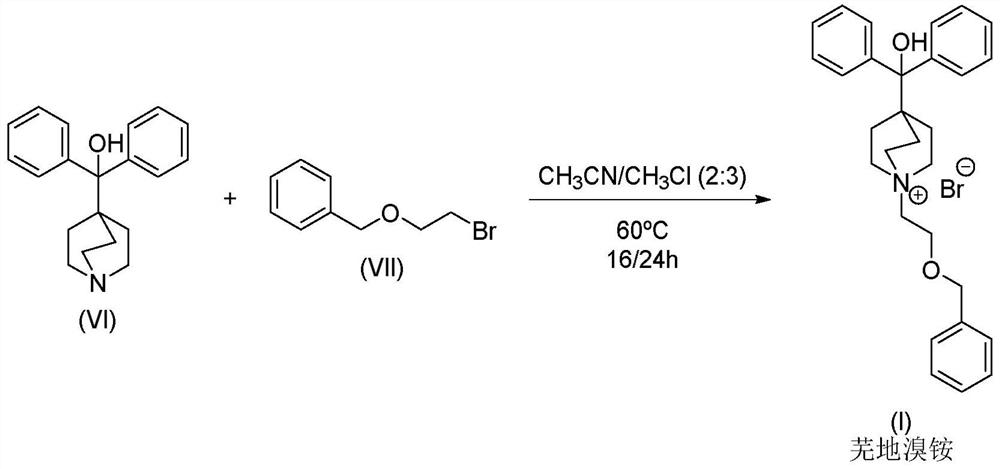
[0064] 1-Azabicyclo[2.2.2]oct-4-yl(diphenyl)methanol (0.3g, 1.0mmol) and ((2-bromoethoxy)methyl)benzene (0.24mL, 1.5mmol) A solution in 1-propanol (30 mL) was injected into a stainless steel coil continuous flow reactor (2.1 mL) at a rate of 0.11 mL / min. The reactor temperature was 180°C. The reaction time was 20 minutes. The solution flowing out from the continuous flow reactor was collected (conversion by HPLC: 93.8%), and the volume was concentrated to 4 mL under reduced pressure. The resulting suspension was cooled to 5°C and stirred for 1 hour. The product was filtered, washed twice with methyl tert-butyl ether (MTBE), and dried under reduced pressure (white powder, 0.34 g, 80%). The product was analyzed by HPLC and a purity of 98.5% was obtained.
Embodiment 2
[0066] Preparation of umeclidinium bromide
[0067] 1-Azabicyclo[2.2.2]oct-4-yl(diphenyl)methanol (0.34g, 1.2mmol) and ((2-bromoethoxy)methyl)benzene (0.27mL, 1.7mmol) A solution in 1-propanol (10 mL) was injected into a stainless steel coil continuous flow reactor (2.1 mL) at a rate of 0.42 mL / min. The reactor temperature was 180°C. The reaction time was 5 minutes. The solution flowing out from the continuous flow reactor (conversion by HPLC: 97.3%) was collected and concentrated to a volume of 4 mL under reduced pressure. The resulting suspension was cooled to 5°C and stirred for 1 hour. The product was filtered, washed twice with methyl tert-butyl ether (MTBE), and dried under reduced pressure (white powder, 0.36 g, 75%). The product was analyzed by HPLC and a purity of 98.9% was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com