Pig platelet lysate and preparation method thereof
A platelet lysate and platelet technology, applied in the field of cell biology, can solve problems such as affecting cell growth and reducing the content of growth factors, and achieve the effects of reducing activation, increasing content and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The invention discloses a preparation method of a platelet lysate and its application. Those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0033] The preparation method of the platelet lysate provided by the present invention and the instruments or reagents used in its application can be purchased from the market.
[...
Embodiment 1
[0054] The preparation of embodiment 1 porcine platelet lysate
[0055] (1) Separation of porcine platelets
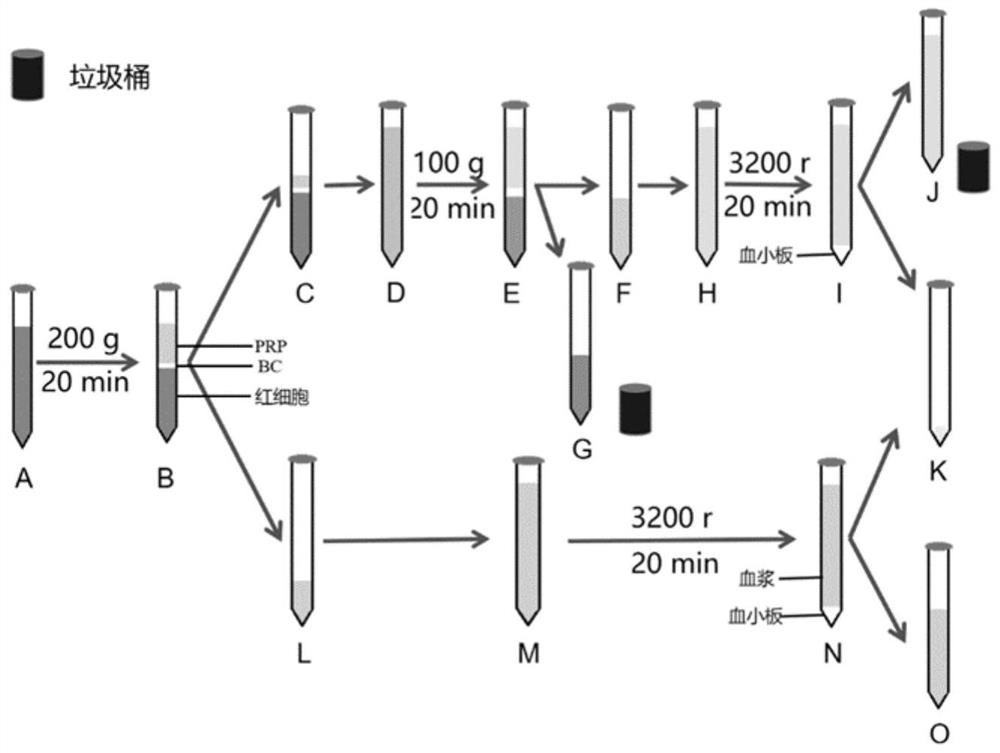
[0056] The specific operation process and its description refer to figure 1 .
[0057] A. Select healthy pigs to be slaughtered, collect blood with coagulation tube, collect serum, and carry out infectious disease detection.
[0058] B. Add 123mL of CPDA-1 anticoagulant to the blood collection bottle in advance, and collect 1L of blood (including anticoagulant) from each pig when slaughtered.
[0059] C. Transfer the collected anticoagulated porcine blood to 15mL centrifuge tube A with a pipette. (When a large number of platelets are separated, the blood can be left to stand or centrifuged at low speed to separate the upper layer into a 15mL centrifuge tube)
[0060] D. Centrifuge the 15mL centrifuge tube A at 200g for 20min at 20-22°C, and you can observe the blood stratification as shown in centrifuge tube B:
[0061] Bottom layer: red blood cells (50%-80% of to...
Embodiment 2
[0079] Determination of endotoxin content in embodiment 2 blood plasma
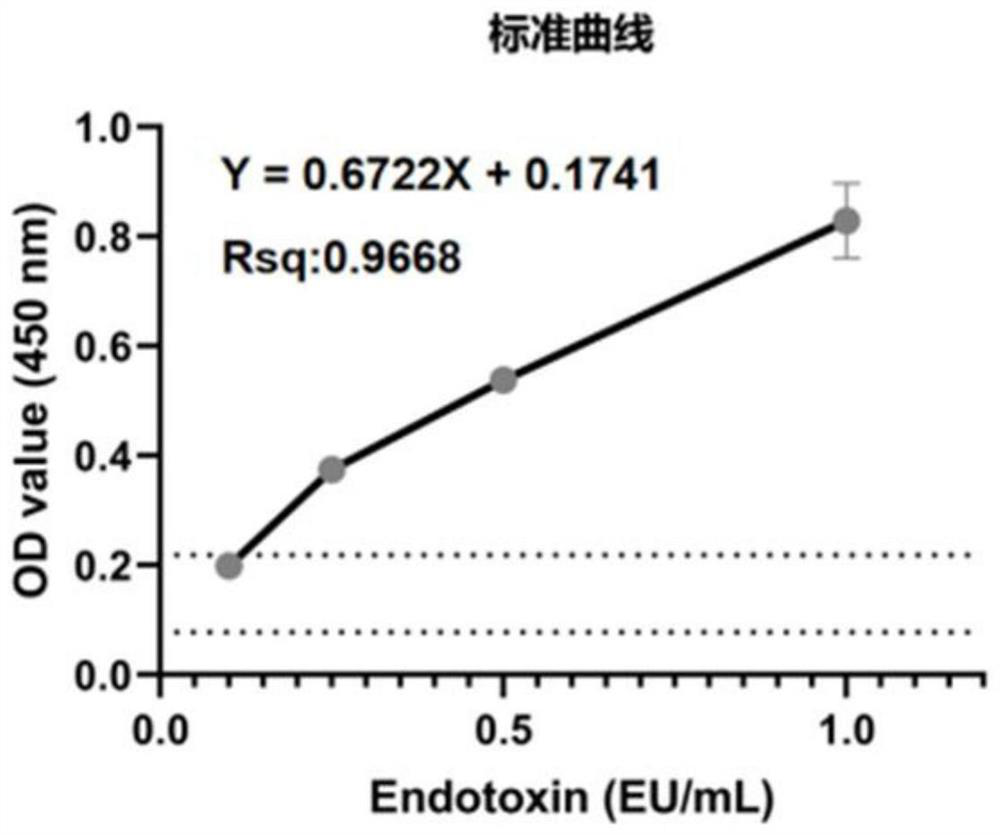
[0080] The endotoxin content in the collected plasma was determined using the endotoxin detection Limulus kit of Xiamen Limulus Reagent Biotechnology Co., Ltd. Make a standard curve of endotoxin with standard products (such as image 3 shown), the OD value at 405nm of 1-9# plasma was measured according to the kit detection method, and the results are shown in Table 3. According to the data in the table and the standard curve, it can be known that the endotoxin content ( image 3 The dotted line in the middle is the actual measured OD value range) are all less than 0.1EU / mL, and the endotoxin content of 8# plasma is 0.06EU / mL according to the standard curve. The endotoxin content of special-grade FBS is usually less than 10EU / mL. In contrast, the endotoxin content in the material collected by the present invention is far lower than the endotoxin content standard of special-grade FBS. It can be inferred t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com