Method of preventing or treating treatment-induced gastrointestinal injury
A gastrointestinal, colonic technology, used in oncology and cancer therapy, medical field, can solve the problem of prevention or treatment of GI damage or complications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1. Materials and methods
[0075] Compound Screening and Preparation
[0076] In silico screening using the ZINC 8.0 database and in silico ADME / toxicity profiles as previously described (Mustata G. et al. "Curr Top Med Chem" (2011) 11:281-290, incorporated by reference incorporated herein) to identify candidate PUMA inhibitor structures and analogs. Compounds related to PUMAi were obtained from commercial suppliers.
[0077] Research design
[0078] The goals of this study were to determine whether PUMA and p53-dependent apoptosis mediate chemotherapy-induced intestinal stem cell loss and enteropathy, and whether their genetic and pharmacological inhibition confers intestinal chemoprotection. Non-tumor-bearing and tumor-bearing mice, p53 and Puma KO mice, small molecule PUMAi, and in vitro mouse and human colon organoids were used to measure the effect of PUMA inhibition on intestinal injury and regeneration, and tumor response following chemotherapy. Sampl...
Embodiment 2
[0134] Example 2. PUMA mediates chemotherapy-induced crypt apoptosis and lethality
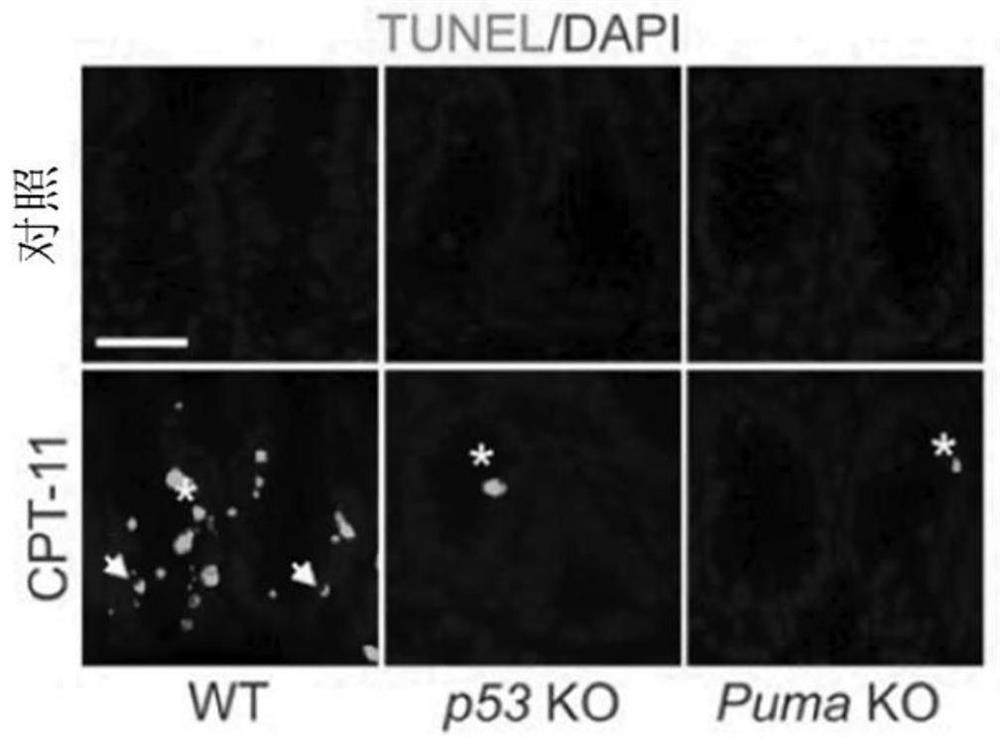
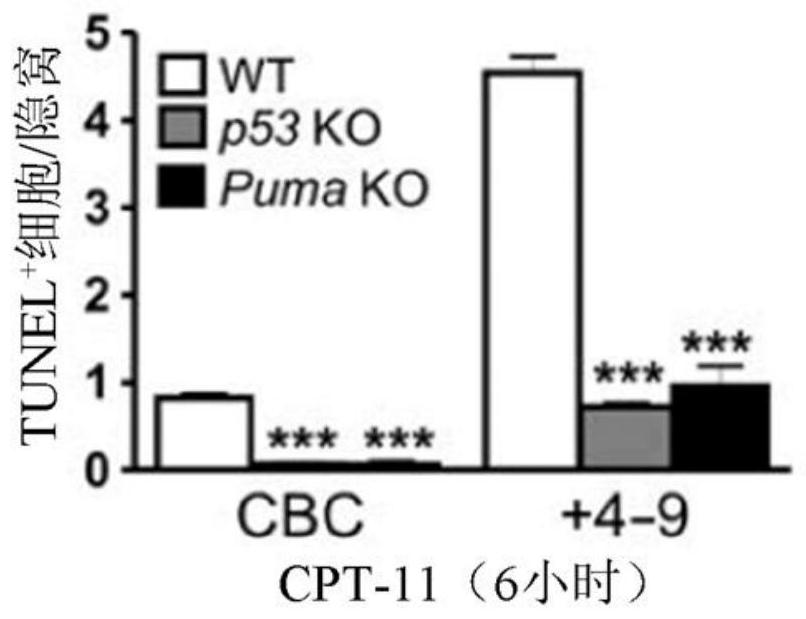
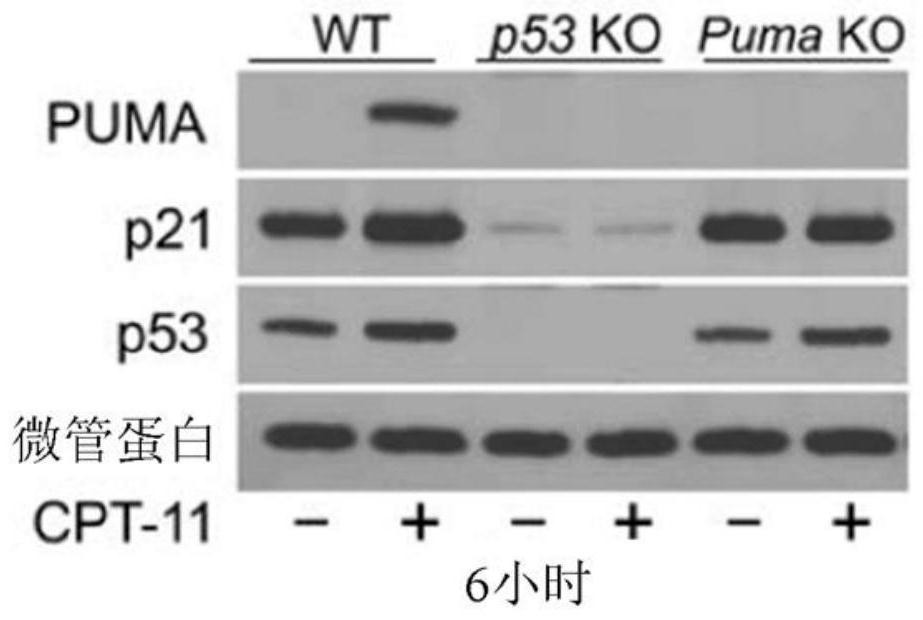
[0135] To investigate the potential role of p53-dependent apoptosis in chemotherapy-induced acute intestinal injury, the inventors treated wild-type (WT), p53 knockout ( KO) and Puma KO mice. CPT-11 induces robust crypt apoptosis in WT mice as measured by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) and active caspase-3 staining , which peaked at 6 hours and gradually decreased through 48 hours. Apoptosis was observed in both stem cells at the base of the crypt (CBCs in positions 1 to 3 relative to the bottom of the crypt) and +4 to 9 cells (relative to the bottom of the crypt), but not in p53 KO and Puma KO mice ( Figure 1A with 1B ,as well as Figures 8A-8D ). CPT-11 treatment induced p53 and its targets PUMA and p21 within 6 hours in the intestinal mucosa of WT but not p53 KO mice ( Figure 1C with Figure 8E ). Puma KO did not...
Embodiment 3
[0136] Example 3. Small molecule PUMAi protects against chemotherapy-induced crypt apoptosis and lethality
[0137] The inventors used pharmacophore modeling of key interactions of the PUMA BH3 domain with BCL-2 family proteins, in silico compound screening, and apoptosis analysis to identify a variety of potential PUMAi. It was confirmed that one of these compounds (PUMAi) inhibited the PUMA / BCL-xL but not the BIM / MCL-1 interaction ( Figures 10A-10C ). In colon cancer cell lines with different p53 or PUMA genotypes, including p53 WT (HCT 116), null (p53 KO), mutants (HT29, SW480, and DLD1) or PUMA null (PUMA KO), PUMAi did not reduce hi CPT-induced apoptosis ( Figure 10D ). PUMA was induced by CPT treatment only in p53 WTHCT116 cells, but not any p53-deficient cell line ( Figure 10E ). and mouse intestinal mucosa ( Figure 1C with 1D ) and very low or undetectable expression in normal human small intestine and colon, the basal amount of PUMA in HCT 116 cells was muc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com