Full-target antigen-presenting cell tumor vaccine as well as preparation method and application thereof
A technology of tumor vaccines and antigens, applied in the field of biomedical engineering, can solve problems such as low efficiency, and achieve the effects of simple preparation process, good application prospects, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
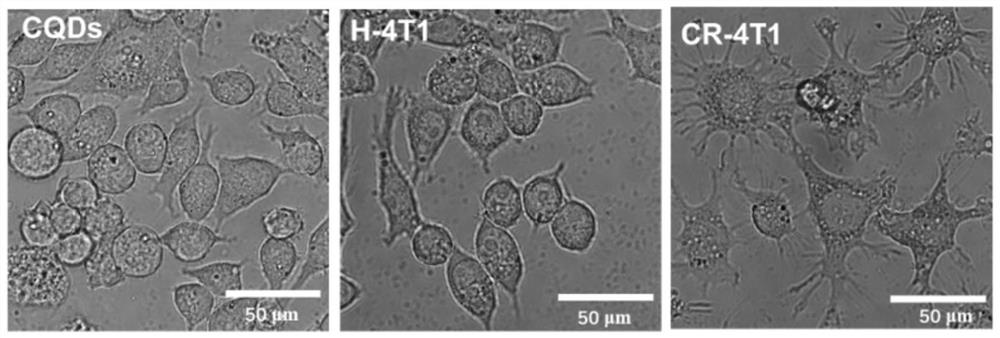
[0034] Some embodiments of the present invention provide a method for preparing an all-target antigen-presenting cell tumor vaccine, which includes: co-incubating quantum dot-modified tumor cells and immature antigen-presenting cells, wherein the quantum dot-modified Tumor cells are obtained by linking quantum dots with protein molecular chains of tumor cells through hydrogen bonds and ligands.
[0035] Specifically, in some embodiments, tumor cells modified with quantum dots are prepared by thermal compounding. The conditions for thermal compounding of quantum dots and tumor cells are as follows: the temperature is 10-100°C, preferably 50-90°C, more preferably 50-80°C, and the compounding time is 1-360min, preferably 1-100min, more preferably 2-30min. For example, heat lamination at 20°C for 200 minutes, or heat lamination at 40°C for 100 minutes, or heat lamination at 80°C for 10 minutes, etc.
[0036] In some embodiments, the thermal compounding process is performed in a s...
Embodiment 1
[0063] This embodiment provides a method for preparing an all-target antigen-presenting cell tumor vaccine, which specifically includes:
[0064] (1) Preparation of carbon quantum dots.
[0065] 1g of citric acid and 3g of urea were dissolved in 30mL of DMSO to obtain a transparent solution, placed in a 50mL polytetrafluoroethylene autoclave, and reacted at 160°C for 4h, and 60mL of ethanol was added to the reacted solution to obtain a black solid, which was washed with water and centrifuged ( 8000rpm, 5min), freeze-dried to obtain dark blue powder, that is, carbon nanodots.
[0066] (2) Thermally compound carbon quantum dots with 4T1 breast cancer cells to obtain quantum dot-modified tumor cells.
[0067] The tumor cell suspension obtained from the shredded tumor tissue obtained from 4T1 breast cancer mice was centrifuged and digested with enzymes supplemented with culture medium; the tumor cell suspension was mixed with inactivated fetal bovine serum, allowed to stand and t...
Embodiment 2
[0071] This embodiment provides a method for preparing an all-target antigen-presenting cell tumor vaccine, which specifically includes:
[0072] (1) Preparation of carbon quantum dots.
[0073] Dissolve 2 g of citric acid and 5 g of urea in 30 mL of DMSO to obtain a transparent solution, place it in a 50 mL polytetrafluoroethylene autoclave, and react for 6 h at 180° C., add 40 mL of ethanol to the reacted solution to obtain a black solid, wash the solid with water, and centrifuge ( 5000rpm, 10min), freeze-dried to obtain dark blue powder, that is, carbon nanodots.
[0074] (2) Thermally compound carbon quantum dots with 4T1 breast cancer cells to obtain quantum dot-modified tumor cells.
[0075] The tumor cell suspension obtained from the shredded tumor tissue obtained from 4T1 breast cancer mice was centrifuged and digested with enzymes supplemented with culture medium; the tumor cell suspension was mixed with inactivated fetal bovine serum, allowed to stand and the cells ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap