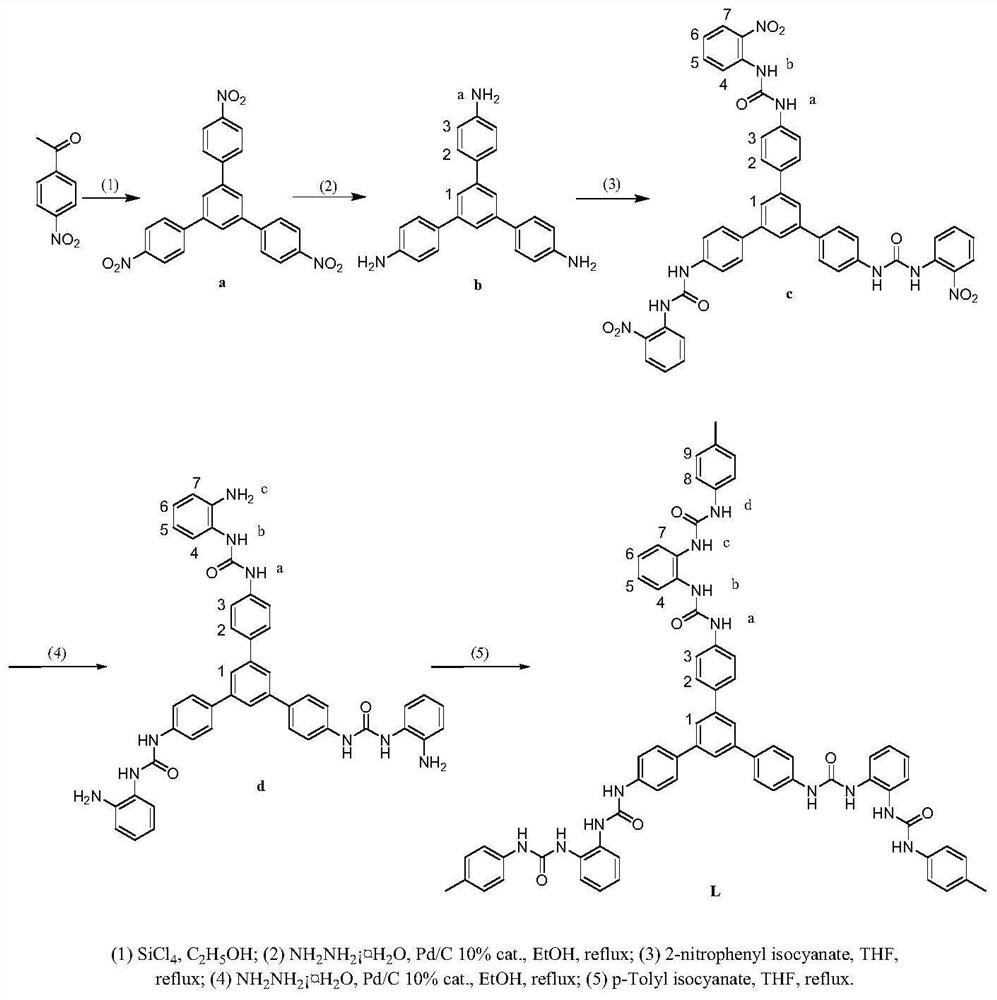
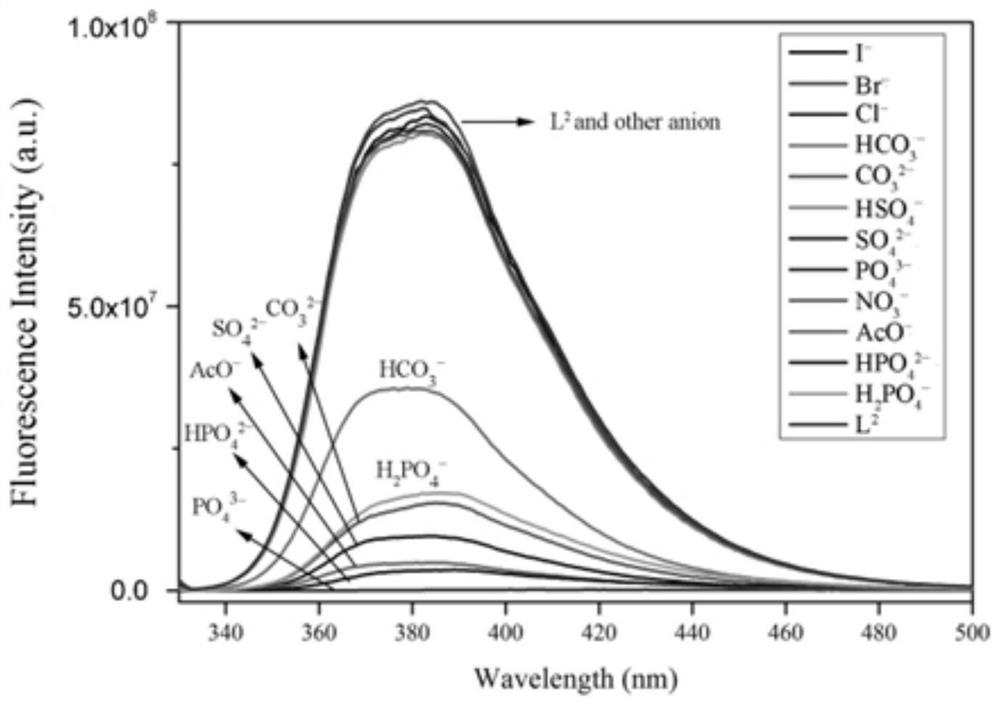
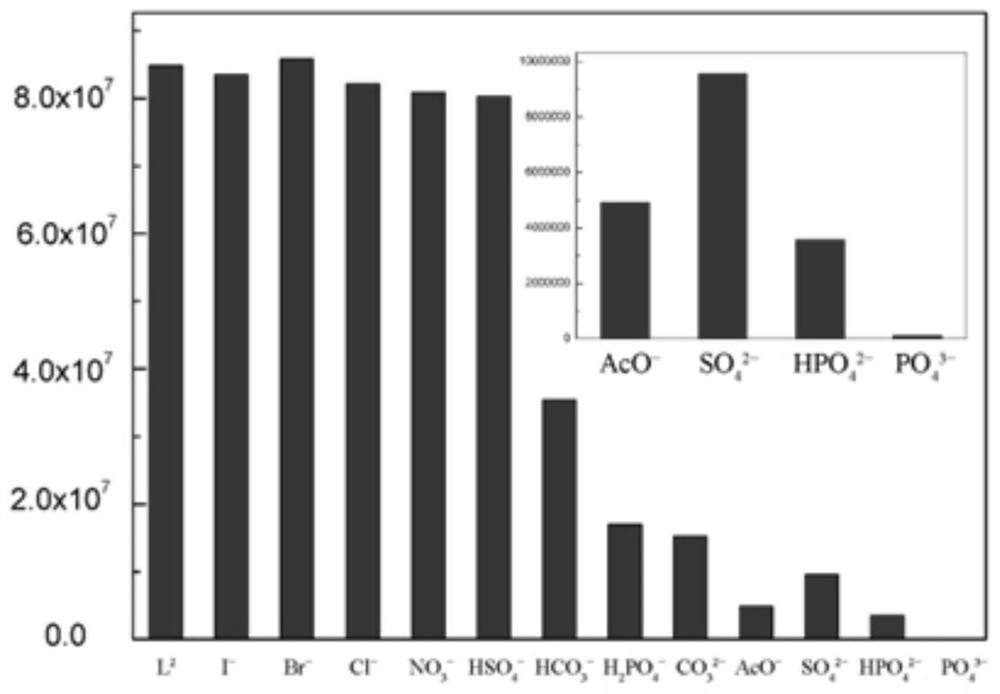
Anion cage compound with fluorescent property and synthesis method thereof
A cage compound and anion technology, applied in the field of anion cage compound and its synthesis, can solve the problems of host-guest properties and application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of compound a:
[0046] Silicon tetrachloride (10mL, 0.09mol) was added dropwise into 40mL dry ethanol solution containing 4-nitroacetophenone. A large number of precipitates were generated immediately, and the mixed solution was refluxed for 10 h. Then cooled to room temperature, the reaction solution was added to 50mL NH 4 Cl aqueous solution, stirred for 10 min. The crude product of compound a was obtained by suction filtration and drying, which was directly used for the preparation of compound b.
Embodiment 2
[0048] Preparation of compound b:
[0049] The crude compound a was directly added to 100 mL of ethanol, and Pd / C 10% (0.4 g, cat.) was added. 80% N was added dropwise to the reaction system under reflux 2 h 4 ·H 2 O (20 mL). After reflux for 10 h, diatomaceous earth was added to remove Pd / C by suction filtration, the filtrate was cooled, and a yellow solid was obtained by suction filtration, which was recrystallized from ethanol and dried in vacuo to obtain compound b as a white solid (2.5 g, 7 mmol, 71%). 1 H NMR (400MHz, DMSO-d 6 ,ppm):δ7.48(s,2H,H3),7.46(s,1H,H1),6.67(d,J=7.6Hz,2H,H2),5.23(s,2H,Ha).ESI-MS :m / z 352.1803[M+H]+
Embodiment 3
[0051] Preparation of compound c:
[0052] Add dropwise 20 mL of b (0.35 g, 1 mmol) dissolved in 15 mL of THF solution dissolved in o-nitroisocyanic acid (0.59 g, 3.6 mmol), reflux for 6 h, cool, filter with suction, wash with toluene and diethyl ether, Compound c was obtained as a yellow solid (0.78 g, 0.93 mmol, 93%). 1 H NMR (400MHz, DMSO-d 6 , ppm): δ10.03(s,1H,Hb),9.67(s,1H,Ha),8.35(d,J=8.8Hz, 1H,H7),8.13(d,J=8.0Hz,1H,H4 ),7.86(s,1H,H1),7.83(s,2H,H3),7.73(t,J=7.4Hz,1H,H5),7.65(d,J=7.6Hz,2H,H2), 7.23( t,J=7.0Hz,1H,H6). 13 C NMR (100MHz, DMSO-d 6 , ppm): 151.81 (CO), 141.07 (C), 138.99 (C), 137.67 (C), 135.06 (C), 134.91 (C), 134.25 (CH), 127.58 (CH), 125.47 (CH), 122.89 (CH),122.59(CH), 122.33(CH),118.86(CH).IR(KBr,ν / cm -1 ):3319(NH),1652(CO), 1585,1498,1435,1340,1276(NO2),1190,1144,829,781,740,648. ESI-MS: m / z 844.2489,[M+H] + ,866.2298,[M+Na]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com