Preparation method of amide
A technology of amides and compound amides, which is applied in the field of amides preparation, can solve the problems of large toxic chemical substances or by-products, and achieve the effects of wide application of substrates, cost reduction, and good development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A preparation method of amide, comprising the following steps:
[0030] The compound of formula (I) and the compound of formula (II) are dissolved in an organic solvent and reacted in an oxygen atmosphere at a temperature of ≥110 ° C to prepare the compound of formula (III) amide; and / or
[0031] The compound of formula (IV) and the compound of formula (II) are dissolved in an organic solvent, and the reaction is carried out at a temperature of ≥110 ° C in an oxygen atmosphere to prepare the compound of formula (III) amide;
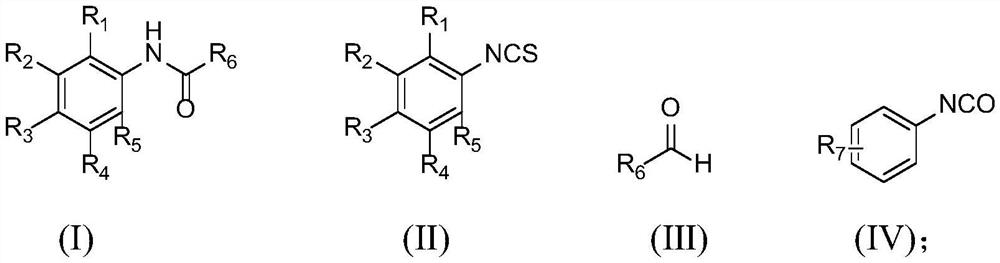
[0032] The structures of the compound of formula (I), compound of formula (II), compound of formula (III) and compound of formula (IV) are as follows:
[0033]
[0034] It can be understood that under the action of oxygen, isothiocyanates or isocyanates and aldehydes can generate amides at temperatures ≥110°C.
[0035] In some of these embodiments, in the preparation method of the amide, the organic solvent is selected from the group consisting...
Embodiment 1
[0055] In a 10 mL sealed tube, add isothiocyanate (0.2 mmol), aldehyde (2 mmol), dichloroethane (0.25 mL, 0.8 M), O 2, and reacted at 115°C for 36h. Monitored by TLC, the reaction was stopped until the isothiocyanate reaction was complete. Extracted with ethyl acetate and water, the organic phase was dried over anhydrous sodium sulfate and spin-dried, the collected crude product was purified by column chromatography [eluent: petroleum ether: ethyl acetate=10:1 (v / v)], Product 1-1 was collected in 86% yield.
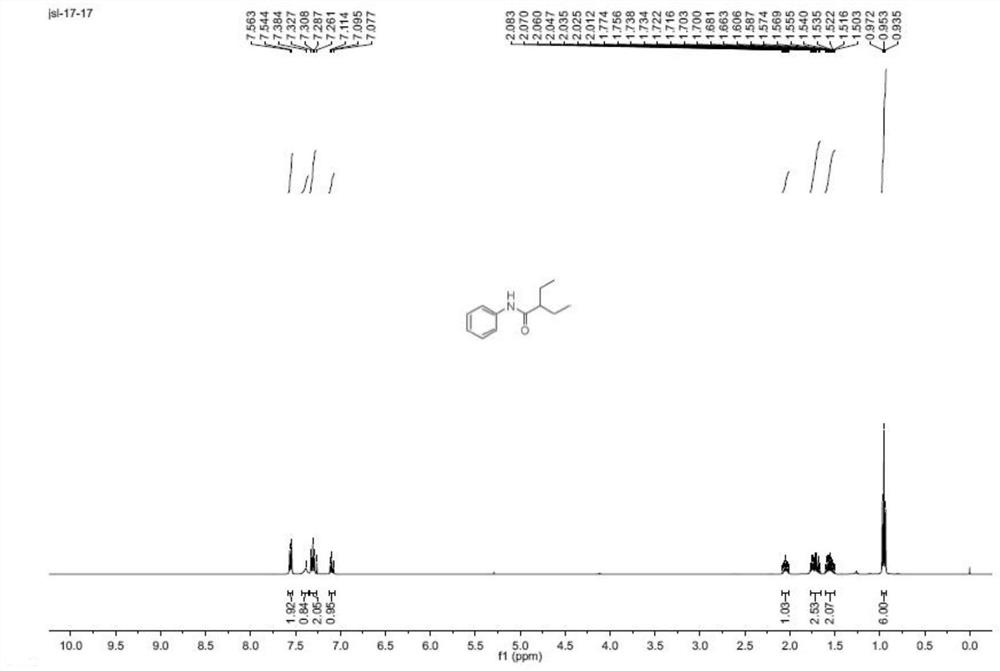
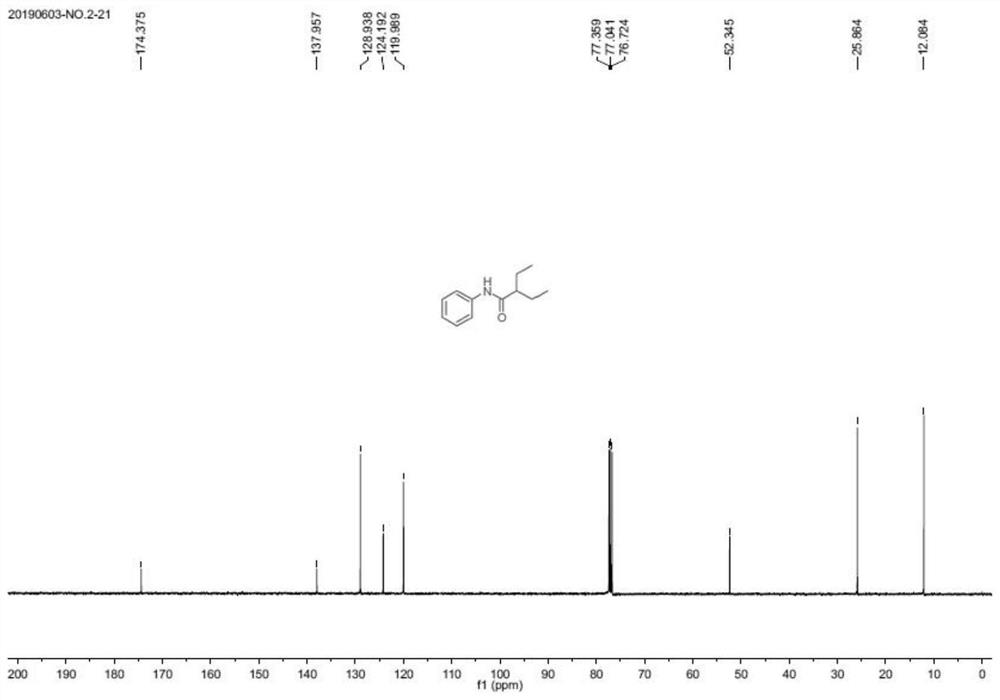
[0056]
[0057] White solid, mp127-128℃. 1 H NMR (400MHz, CDCl 3 )δ7.55(d,J=7.7Hz,2H),7.38(s,1H),7.31(t,J=7.9Hz,2H),7.10(t,J=7.4Hz,1H),2.08–2.01( m, 1H), 1.77–1.66 (m, 2H), 1.61–1.50 (m, 2H), 0.95 (t, J=7.4Hz, 6H). 13 C NMR (100 MHz, CDCl 3 )δ174.4,138.0,129.0,124.2,120.0,52.4,25.9,12.1.HRMS(ESI): Calcd for C 12 H 18 NO[M+H] + :192.13829;Found:192.13796.
[0058] Yield=molar amount of compound of formula (I-1) / molar amount of phenyl isothiocyanate×100%
Embodiment 2
[0060] Basically the same as Example 1, only the volume of dichloromethane added is different, that is, the concentration is different.
[0061] Phenyl isothiocyanate (0.2 mmol), 2-ethylbutanal (2 mmol), different volumes of dichloroethane (different concentrations) (0.1, 0.3, 0.5, 0.8, 1.0 M) were added to 10 mL of resistant In the pressure tube, O 2 , and reacted at 115 °C for 36 h. Monitored by TLC, the reaction was stopped until the isothiocyanate reaction was complete. Extracted with ethyl acetate and water, the organic phase was dried over anhydrous sodium sulfate and spin-dried, the collected crude product was purified by column chromatography [eluent: petroleum ether: ethyl acetate=10:1 (v / v)], The coupling product I-1 is isolated, and the calculated separation yield is shown in Table 1.
[0062] Table 1 Effects of different concentrations on the coupling reaction
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com