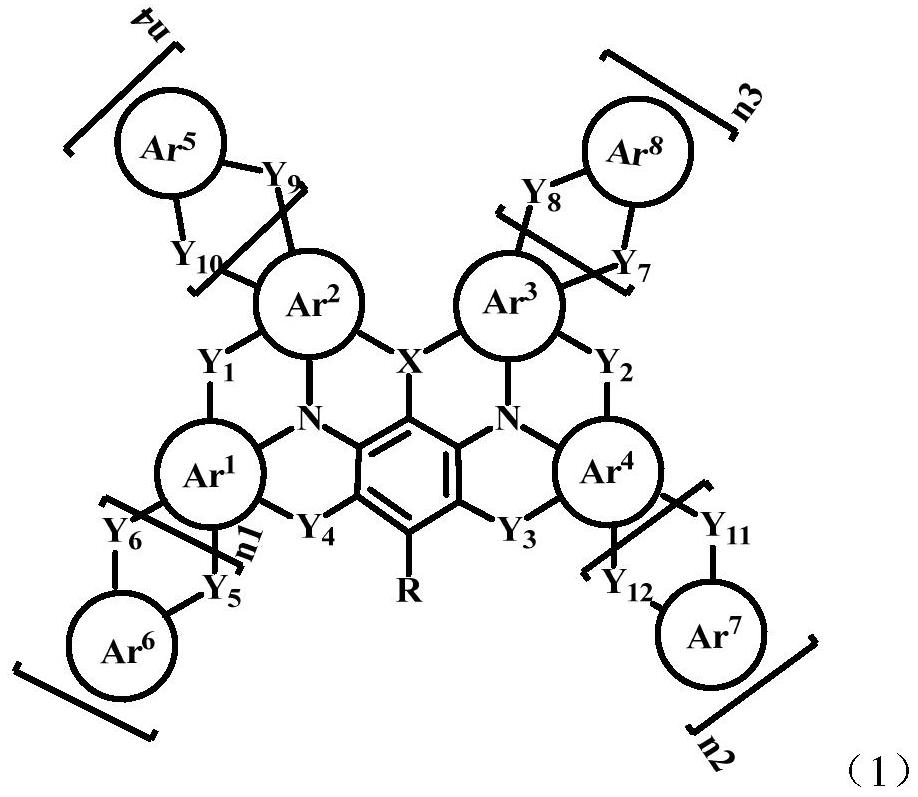
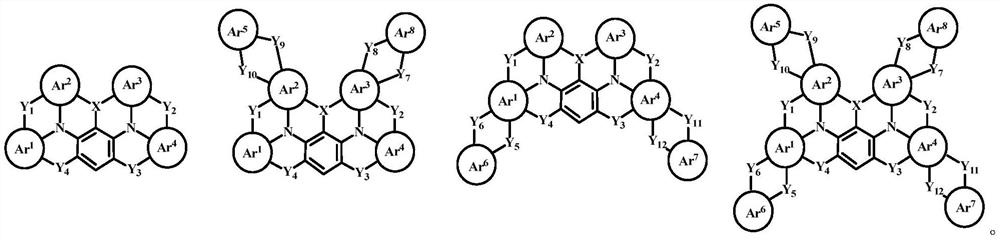
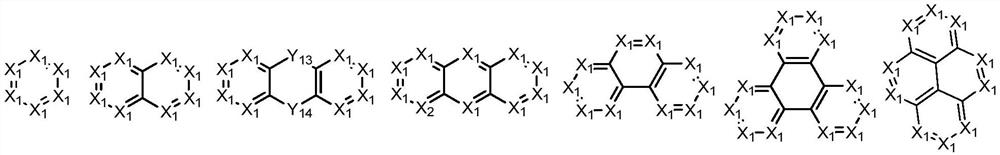
N-containing fused ring compound and application thereof in organic electronic device
A technology of organic compounds and ring atoms, applied in the field of electroluminescent materials, can solve the problems of stability and insufficient rigidity of luminescent host materials, and achieve the effects of improving luminous efficiency and life, reducing roll-off and improving rigidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] The synthetic route of compound (1) is as follows:
[0168]
[0169]
[0170] Synthesis of intermediate 3:
[0171] Under nitrogen protection atmosphere, in a dry three-necked flask, add 1mmol of intermediate 1 and 2.1mmol of intermediate 2 respectively, pour 100ml of DMSO as a solvent, add dry K 2 CO 3 As a base, react at 120°C for 8 hours, monitor the reaction by TLC, after the reaction is complete, cool the reaction solution to room temperature, add water and dichloromethane in sequence, wash the reaction solution with water for several times, and extract the aqueous phase with dichloromethane Multiple times, the organic phases were combined and washed with anhydrous Na 2 CO 3 Dry, filter, and spin dry the reaction solution to obtain a crude product, which is recrystallized from ethyl acetate to obtain 0.78 mmol of intermediate 3, with a reaction yield of 78%, MS (ASAP) = 960.7.
[0172] Synthesis of intermediate 4:
[0173] Under nitrogen protection atmos...
Embodiment 2
[0176]
[0177] The synthetic route of compound (2) is as follows:
[0178]
[0179] Synthesis of intermediate 3:
[0180] The synthesis method is exactly the same as the synthesis method of intermediate 3 in compound (1), except that the raw material is changed from bromocarbazole to bromonaphthalene carbazole (intermediate 2), and the yield is 82.1%. MS (ASAP) = 1127.1.
[0181] Synthesis of Intermediate 4:
[0182]The synthesis method is exactly the same as the synthesis method of intermediate 4 in compound (1), and the yield is 30.5%. MS (ASAP) = 807.5.
[0183] Synthesis of intermediate 5:
[0184] In a 1000ml bottle, add intermediate 4 (57mmol), reduced iron powder (239.4mmol), ammonium chloride (239.4mmol) and 10ml of concentrated hydrochloric acid and 800ml of MeOH / THF / H 2 O mixed solvents were heated to 70° C. to react in the air environment, and the reaction was tracked by TLC. After the reaction is complete, cool to room temperature, remove the solvent i...
Embodiment 3
[0188]
[0189] The synthetic route of compound (3) is as follows:
[0190]
[0191] Synthesis of Intermediate 3:
[0192] The synthesis method is exactly the same as the synthesis method of intermediate 3 in compound (1), the solvent is changed from DMSO to DMF, and the yield is 83.4%. MS (ASAP) = 1161.0.
[0193] Synthesis of Intermediate 4:
[0194] The synthesis method is exactly the same as the synthesis method of intermediate 4 in compound (1), and the yield is 28.5%. MS (ASAP) = 841.4.
[0195] Synthesis of compound (3):
[0196] The synthesis method is exactly the same as that in compound (1), and the yield is about 31.2%. MS (ASAP) = 612.5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com