Benzofuran derivative as well as preparation method and application thereof
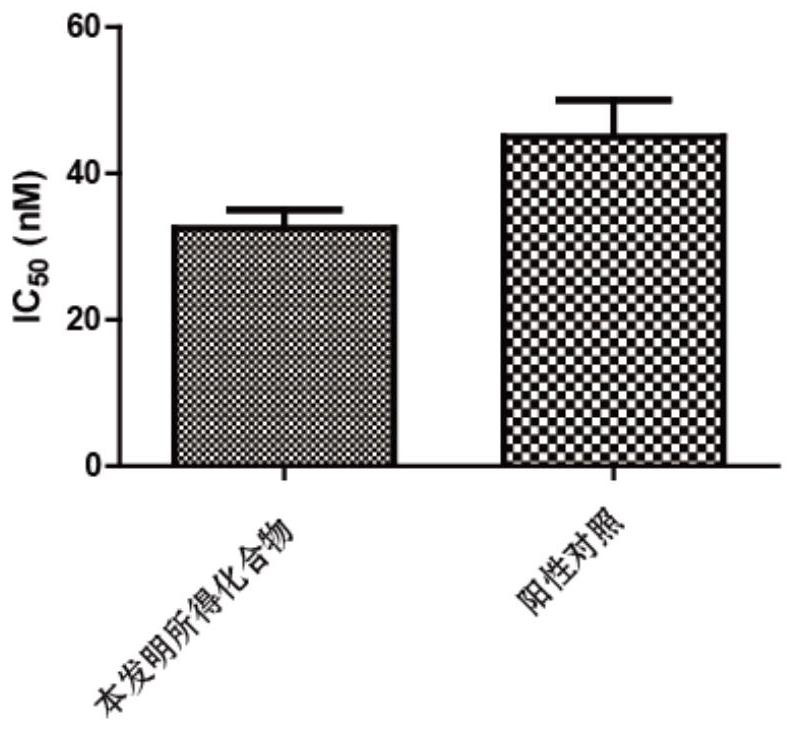
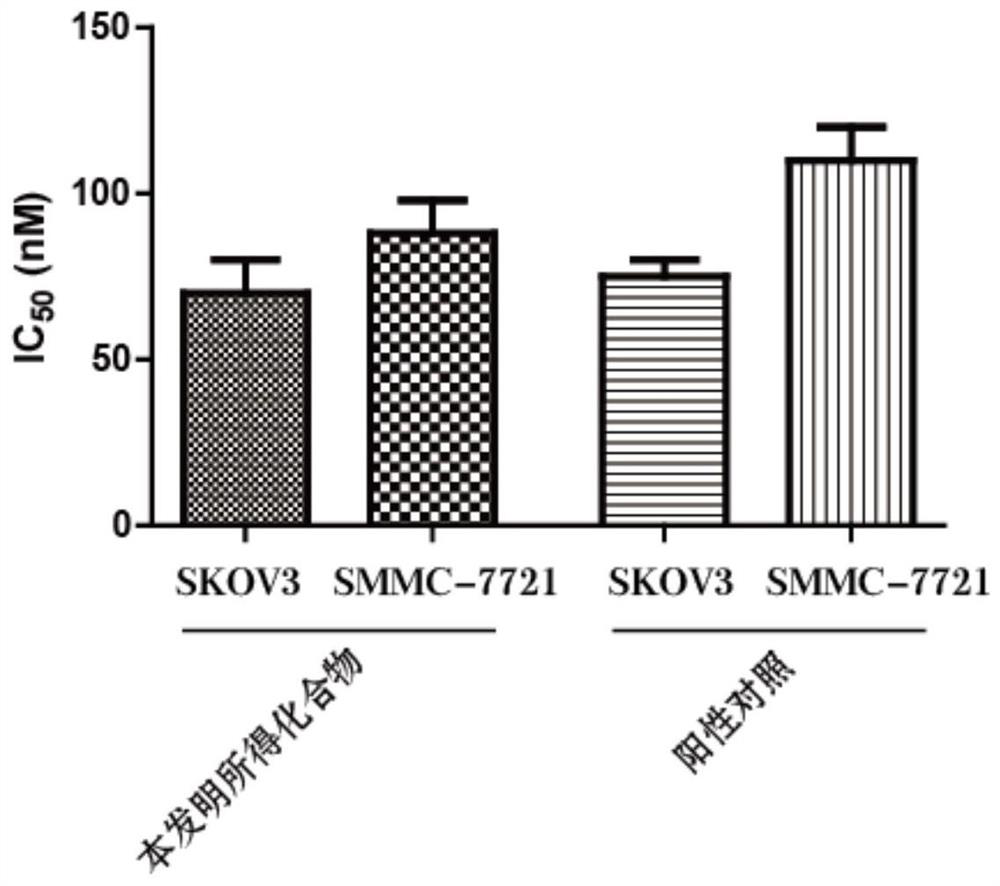
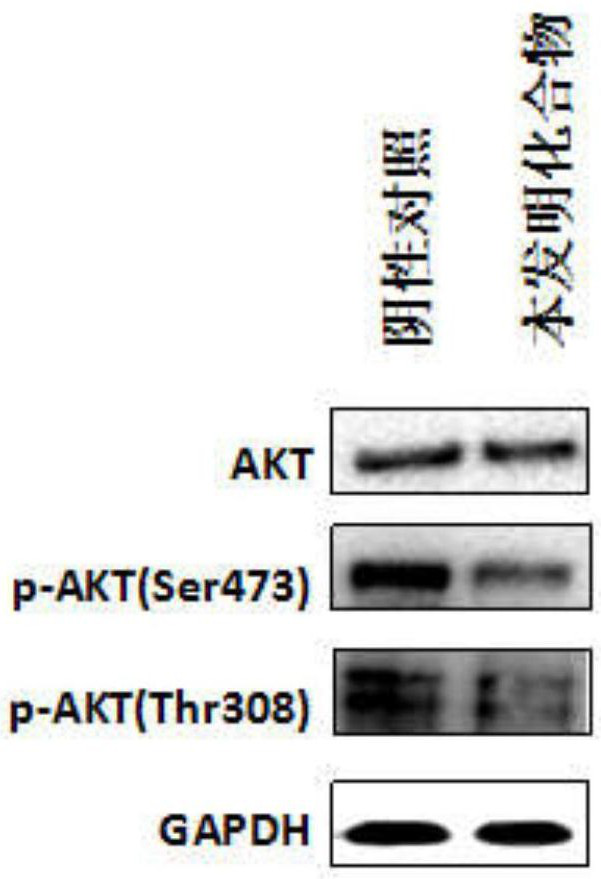
A technology of benzofuran and derivatives, which is applied in the field of benzofuran derivatives and their preparation, can solve the problems of unseen, small toxic and side effects, strong targeting effect, etc., and achieve good inhibitory effect and good AKT protein kinase Inhibitory effect, strong effect of tumor suppressor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. Preparation of compound 1
[0034]
[0035] (1) Intermediate 2,3-bis((bromotriphenyl-λ 5 Synthesis of -phosphoryl)methyl)benzene-1,4-diol (compound shown in structural formula 2):
[0036] 2,3-Bis(bromomethyl)benzene-1,4-diol (compound of formula 1) (100 mmol) and triphenylphosphine (210 mmol) were heated under reflux in 160.0 mL of chloroform for 0.5 hours. The reaction mixture was then allowed to cool and the white precipitate formed was filtered off. The filtrate was evaporated to dryness in vacuo, the obtained crude product was dissolved in 50.0 mL of toluene to be slurried, filtered, washed with toluene, the resulting solids were combined and dried under vacuum at 50°C to obtain 66.46 g of an off-white solid with a yield of 81% .
[0037] 1 H-NMR (400MHz, CDCl 3 )δ: 2.60(d, 4H), 6.49(s, 2H), 7.15(m, 12H), 7.44-7.47(m, 18H), 9.68(s, 2H). 13 C-NMR (125MHz, CDCl 3 )δ: 29.58, 118.00, 123.17, 126.49, 131.37, 132.66, 134.54, 147.30.
[0038] (2) Synth...
Embodiment 2
[0050] Example 2. Preparation of compound 2 (compound represented by chemical structural formula 7)
[0051] (1)(2,6-Dibutylbenzo[1,2-b:5,4-b']difuran-3,5-diyl)bis((4-((2-methpropenyl) ) oxy) phenyl) ketone) (compound shown in chemical structure 7) synthesis:
[0052]
[0053] Potassium carbonate (20 mmol) was added to (2,6-dibutylbenzo[1,2-b:5,4-b']difuran-3,5-diyl)bis((4-hydroxyphenyl) ) ketone) (compound of formula 5) (20 mmol) in toluene (80 mL). The mixture was then stirred at 25-30°C for 0.5 hours and heated to reflux, after which 3-chloro-3-methylpropene (45 mmol) was added maintaining the temperature. The reaction mixture was refluxed for 5 hours. The reaction mixture was then cooled to 25-30°C, and the inorganic solids were filtered and washed with toluene (50 mL). The filtrate was collected and concentrated under reduced pressure, followed by flash column chromatography (ethyl acetate as the eluent) to give 9.71 g of an off-white solid in 78.5% yield.
[0054...
Embodiment 3
[0055] Example 3. Preparation of compound 3 (compound represented by structural formula 8)
[0056] (1)(2,6-Dibutylbenzo[1,2-b:5,4-b']difuran-3,5-diyl)bis((4-(2-methylbutoxy) ) phenyl) ketone) (compound of formula 8) synthesis
[0057]
[0058]Potassium carbonate (20 mmol) was added to (2,6-dibutylbenzo[1,2-b:5,4-b']difuran-3,5-diyl)bis((4-hydroxyphenyl) ) ketone) (compound of formula 5) (20 mmol) in toluene (80 mL). The mixture was then stirred at 25-30°C for 0.5 hours and heated to reflux, after which 1-chloro-2-methylbutane (45 mmol) was added maintaining the temperature. The reaction mixture was refluxed for 5 hours. The reaction mixture was then cooled to 25-30°C, and the inorganic solids were filtered and washed with toluene (50 mL). The filtrate was collected and concentrated under reduced pressure, followed by flash column chromatography (ethyl acetate as the eluent) to give 10.74 g of an off-white solid in 82.5% yield.
[0059] 1 H-NMR (400MHz, CDCl 3 )δ: 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com