Application of hematoporphyrin derivative combined with chemical drug in breast cancer treatment
A kind of derivative, hematoporphyrin technology, which is applied in the field of medicine to increase the sensitivity of chemotherapeutic drugs, can solve the problems of low polarity of hematoporphyrin and limited application, and achieves the effect of broad application prospect and easy-to-mastery method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Synthesis of hematoporphyrin derivative A2
[0041]Substrates, Torp porphyr derivatives A2 by amide condensation reaction by amide condensation reaction by amide condensation reaction by catalyzing hemptidin and Vivaimi fragment (3,4-dimethoxyethylenethylamine). The synthetic process is as follows:
[0042]
[0043]The specific operation is:
[0044]0.5 mmol hemolin is dissolved in a reaction vessel having 30 ml of dimethylformamide (DMF), and 4 mmol3,4-dimethoxytinethylamine and 0.5 mmol benzotriazole-1-oxygen were added. Basis (dimethylamino) phosphonium hexafluorophosphate (BOP), at room temperature for 3 hours, TLC detection reaction process; after the reaction is completed, 100 ml of water is added to the reaction vessel, and the reaction vessel is placed on ice waiting Preferred solids; extracting the solid and dried, i.e. to obtain a crude product; dissolve the crude product in ethyl acetate, add 200-300 weeks of silicate silicate, vacuum rotation, evaporation of ethyl acetate In s...
Embodiment 2
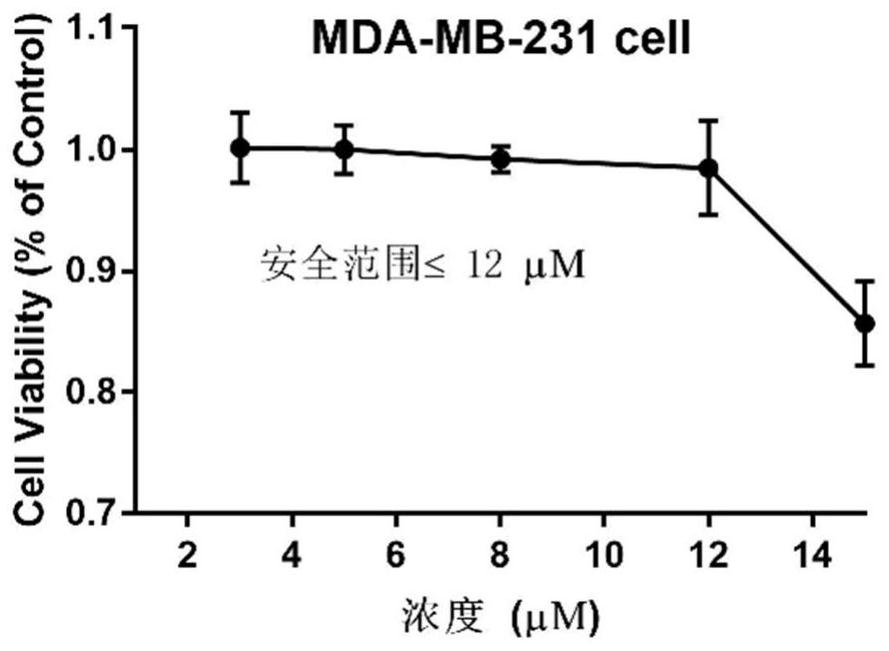
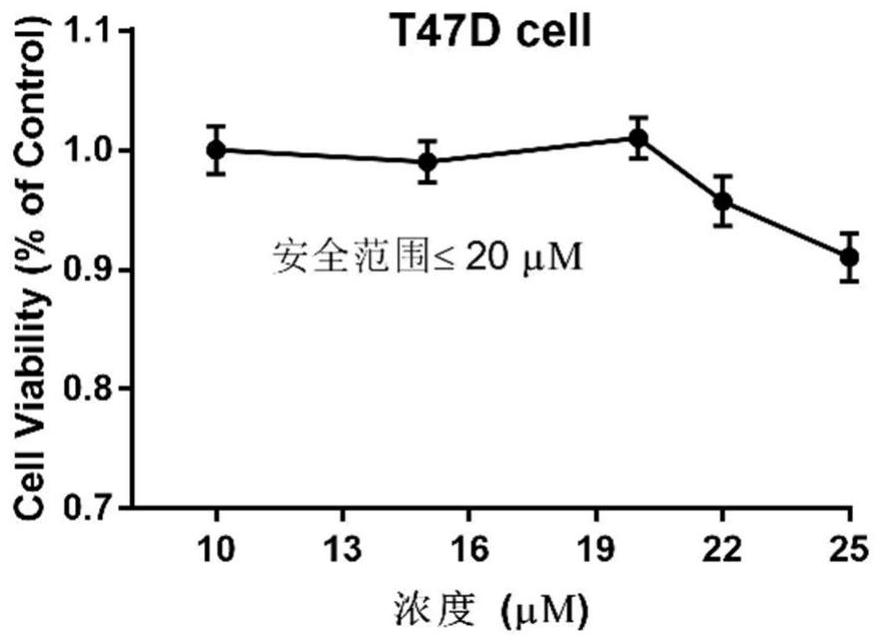
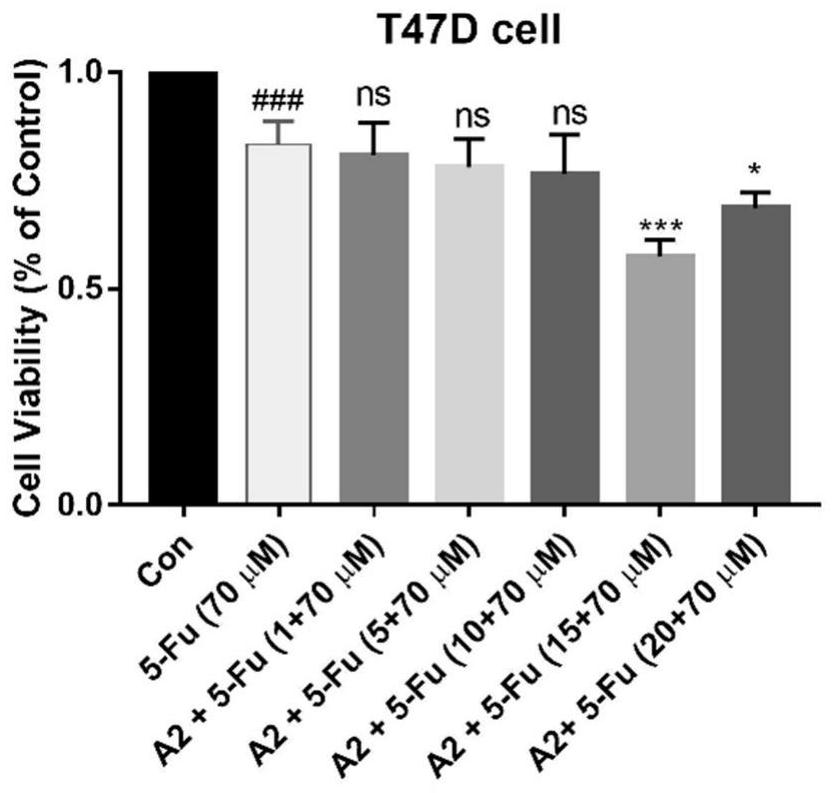
[0048]CCK8 kit detection testing hematoporphyrin derivative A2 and chemotherapeutic drug inhibits breast cancer cell value-added activity
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com