Application of hematoporphyrin derivative in treatment of bladder cancer together with chemical drug
A derivative, the technology of hematoporphyrin, applied in the field of medicine to increase the sensitivity of chemotherapeutic drugs, can solve the problems of limited application and low polarity of hematoporphyrin, and achieve the effect of easy method and broad application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of Hematoporphyrin Derivative A2
[0038] Hematoporphyrin and verapamil fragments (3,4-dimethoxyphenethylamine) are catalyzed by BOP to synthesize the target product, namely hematoporphyrin derivative A2, through amide condensation reaction. The synthesis process is as follows:
[0039]
[0040] The specific operation is:
[0041] Dissolve 0.5 mmol of hematoporphyrin in a reaction vessel filled with 30 mL of dimethylformamide (DMF), and add 4 mmol of 3,4-dimethoxyphenethylamine and 0.5 mmol of benzotriazol-1-yloxy base tris(dimethylamino)phosphonium hexafluorophosphate (BOP), stirred at room temperature for 3 hours, and TLC detected the reaction progress; Precipitate a solid; suction filter the precipitated solid and dry to obtain a crude product; dissolve the crude product in ethyl acetate, add 200-300 mesh silica gel equivalent to three times the mass of the crude product, and vacuum rotary evaporate ethyl acetate ; In silica gel column chromatography ...
Embodiment 2
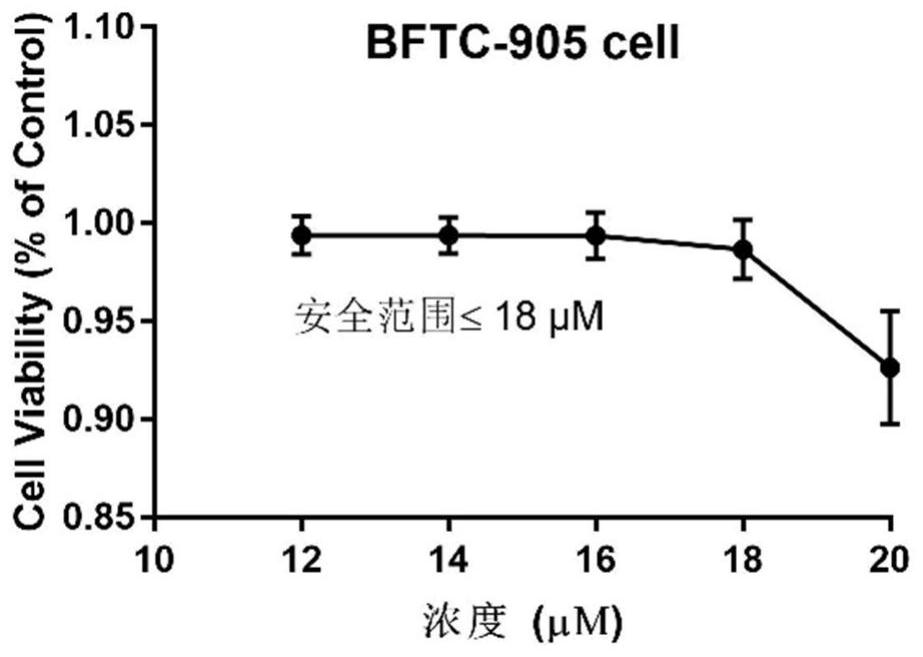
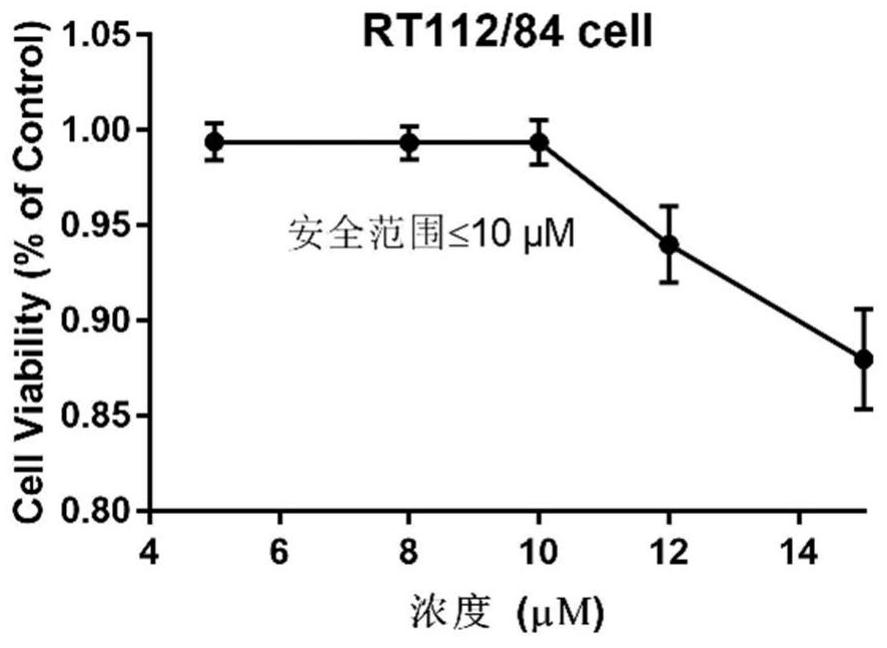
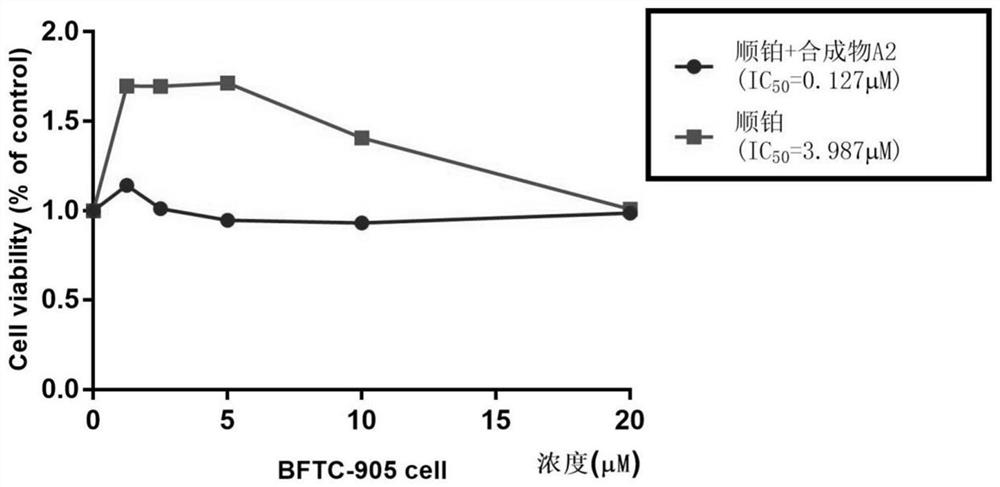
[0045] CCK8 kit detects hematoporphyrin derivative A2 and chemotherapeutic drugs to inhibit proliferation of bladder cancer cells
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com