An organic photoelectric functional material and an organic electroluminescent device prepared by using the same
A photoelectric functional material and organic technology, applied in luminescent materials, electric solid devices, organic chemistry, etc., can solve problems such as short life and restrictions on the application of OLED white light devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0078] Table 1. Preparation of Example Product Data Summary:
[0079]
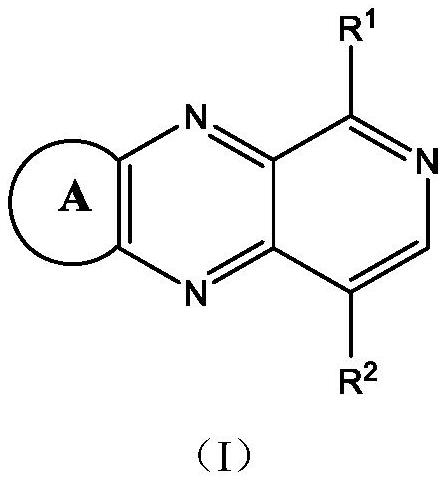
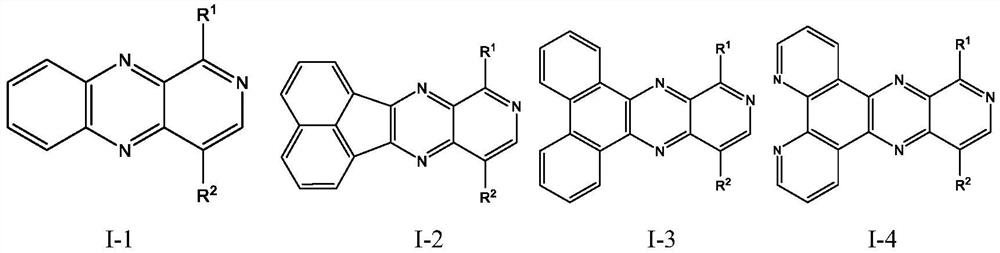
[0080] 1,2-benzoquinone or acenaphthenequinone or phenanthrenonequinone or 1,10-phenanthroline-5,6-dione and 2-bromopyridine-3,4diamine and / or 5-bromopyridine-3,4 Diamine was added in acetic acid according to the molar ratio of 1:1, refluxed for 6 hours, and after cooling to room temperature, the reaction solution was poured into water, filtered, and the filter cake was washed with water and methanol respectively, and the gained filter cake was vacuum-dried to obtain 1 - and / or 4-bromobenzopyridopyrazines.
[0081] The 1- and / or 4-bromobenzopyridopyrazine and the intermediate reactant are reacted in one step or two steps of the same type according to the molar ratio of 1:1.1 under the protection of nitrogen, in tetrahydrofuran and water with potassium carbonate, tetrahydrofuran (Triphenylphosphine) palladium was refluxed for 24 hours, then cooled to room temperature, the reaction solution was filtered,...
preparation Embodiment 2
[0094] Table 2. Preparation of Example Product Data Summary:
[0095]
[0096]
[0097]
[0098]
[0099] 1,2-benzoquinone or acenaphthenequinone or phenanthrenonequinone or 1,10-phenanthroline-5,6-dione and 2-bromopyridine-3,4diamine and / or 5-bromopyridine-3,4 Diamine was added in acetic acid according to the molar ratio of 1:1, refluxed for 6 hours, and after cooling to room temperature, the reaction solution was poured into water, filtered, and the filter cake was washed with water and methanol respectively, and the gained filter cake was vacuum-dried to obtain 1 - and / or 4-bromobenzopyridopyrazines.
[0100] The 1- and / or 4-bromobenzopyridopyrazine and the intermediate reactant are reacted in one step or two steps of the same type according to the molar ratio of 1:1.1 under the protection of nitrogen, in tetrahydrofuran and water with potassium carbonate, tetrahydrofuran (Triphenylphosphine) palladium was refluxed for 24 hours, then cooled to room temperature,...
preparation Embodiment 3
[0113] Table 3. Preparation of Example Product Data Summary:
[0114]
[0115]
[0116]
[0117]
[0118] 1,2-benzoquinone or acenaphthenequinone or phenanthrenonequinone or 1,10-phenanthroline-5,6-dione and 2-bromopyridine-3,4diamine and / or 5-bromopyridine-3,4 Diamine was added in acetic acid according to the molar ratio of 1:1, refluxed for 6 hours, and after cooling to room temperature, the reaction solution was poured into water, filtered, and the filter cake was washed with water and methanol respectively, and the gained filter cake was vacuum-dried to obtain 1 - and / or 4-bromobenzopyridopyrazines.
[0119] 1- and / or 4-bromobenzopyridopyrazine and the intermediate reactant are reacted in one step or two steps of the same type according to the molar ratio of 1:1.1 under the protection of nitrogen, in o-dichlorobenzene with carbonic acid Cesium, bis(dibenzylideneacetone)palladium and tri-tert-butylphosphine were added and heated to reflux for 24 hours, then coo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com