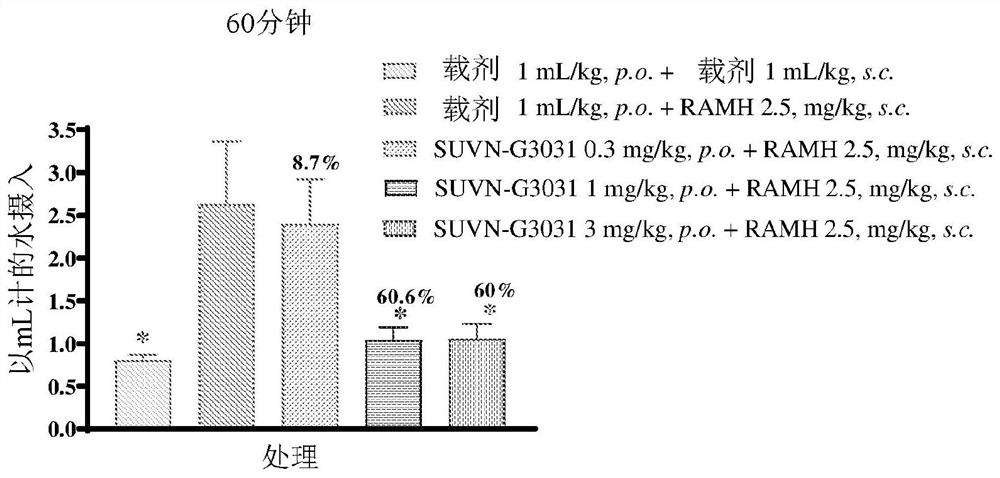
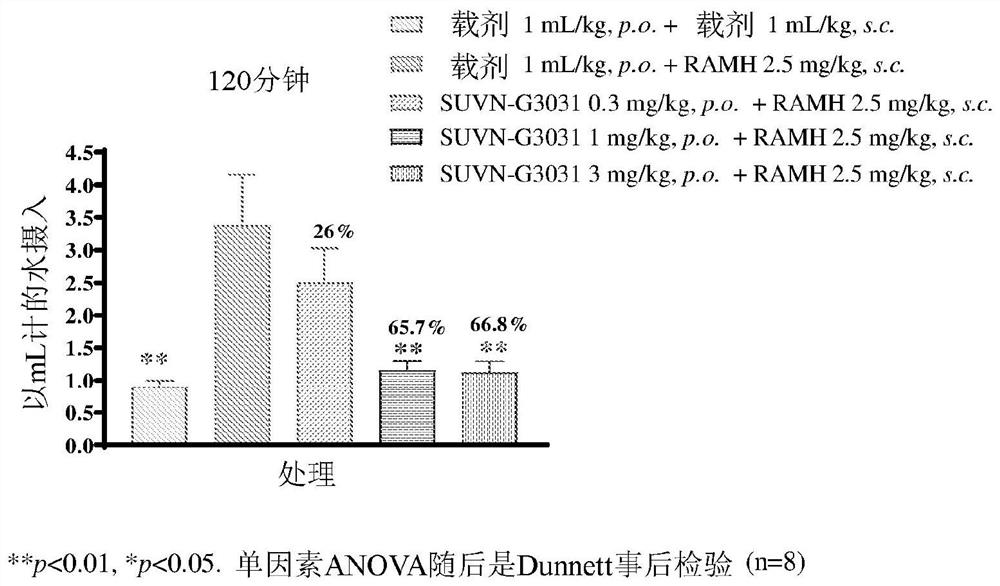
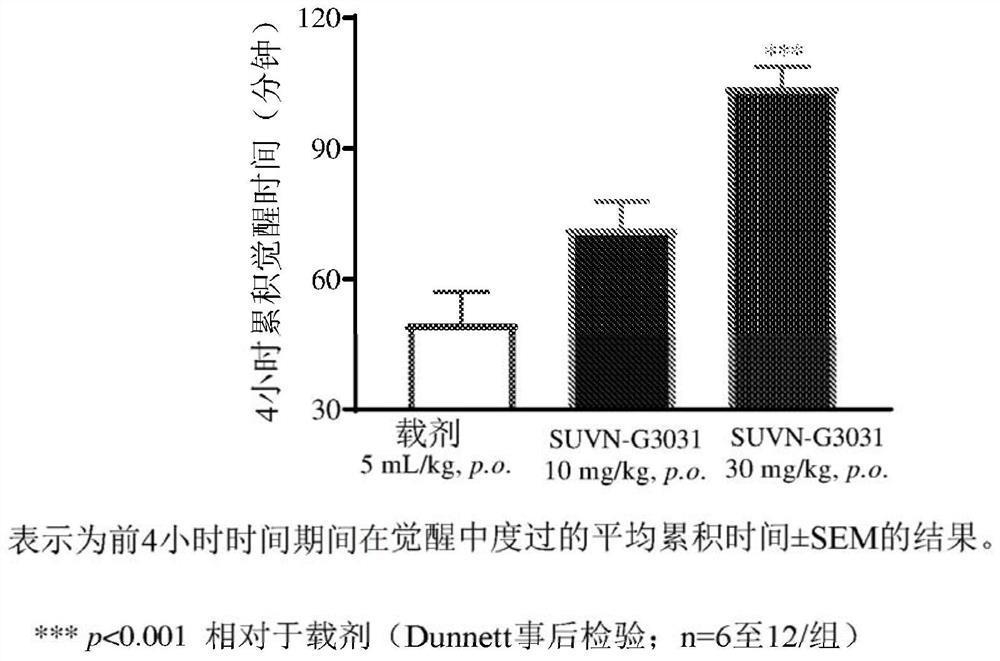
Method of treatment with histamine-3 receptor inverse agonist
An inverse agonist, histamine technology, applied in the field of quantitative histamine-3, can solve the problems of reduced quality of life of narcolepsy individuals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0063] The present invention encompasses, but is not limited to, all of the embodiments described herein, however, some preferred aspects and elements of the invention are discussed herein in the form of the following embodiments.
[0064] In one embodiment, the invention relates to the treatment of narcolepsy, excessive daytime sleepiness, obstructive sleep apnea, circadian rhythm sleep disorder, associated with Parkinson's disease, multiple sclerosis, dementia or attention deficit hyperactivity disorder A method of sleep and alertness disorders comprising administering to a patient in need thereof a therapeutically effective amount of a histamine-3 receptor inverse agonist, wherein the histamine-3 receptor inverse agonist is N-[4- (1-cyclobutylpiperidin-4-yloxy)phenyl]-2-(morpholin-4-yl)acetamide or a pharmaceutically acceptable salt thereof.
[0065] In another embodiment, the present invention is directed to a method of treating narcolepsy comprising administering to a pat...
Embodiment 1
[0178] Example 1: K in the context of human and rat histamine-3 receptors i Determination of value:
[0179] Histamine-3 membranes were prepared from recombinant CHO-k1 cells expressing human histamine-3 receptor or rat histamine-3 receptor. Radioligand (RS)-[alpha]-methylhistamine [cyclo-1,2-3H] was purchased from American Radiolabeled chemicals (ARC) (Catalog No. ART 1342). Final ligand concentration was 3 nM; non-specific determinants were methylhistamine (100 μM) and histamine-3 membrane (60 μg / well). Methylhistamine was used as a positive control. at 25°C in a solution containing 5 mM MgCl 2 The reaction was carried out in a binding buffer of 50 mM Tris-HCl (pH 7.4) for 60 minutes. The incubation was terminated by rapid filtration, followed by washing the binding mixture 4 times using 96-well harvest plates (Millipore, cat# MSFCNXB50) pre-coated with 0.5% polyethyleneimine. Plates were dried and bound radioactivity collected on the filters was determined by scintilla...
Embodiment 2
[0183] Example 2: IC in the context of the human histamine-3 receptor 50 Determination of value:
[0184] Compounds were tested under Eurofins Cerep Panlabs according to the following procedure.
[0185] Materials and methods:
[0186] Source of receptor: human recombinant expressed in CHO-K1 cells
[0187] Radioligand: [35S]-GTPγS
[0188] Vehicle: 1% DMSO
[0189] Reference agonist: R(-)-α-methylhistamine
[0190] Agonist concentration: 30nM
[0191] Positive control: Thioperamide
[0192] Incubation conditions: at 30°C in a medium containing 100mM NaCl, 10mM MgCl 2 , 1 mM DTT, 1 mM EDTA in 20 mM HEPES (pH 7.4) for 30 minutes. After incubation, scintillation is measured on a luminescence counter and compared to control values in order to determine any interaction of the test compound with the cloned histamine-3 binding site.
[0193] result:
[0194] The compound SUVN-G3031 exhibits the functional properties of an inverse agonist. IC 50 The values are tabulat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com