Hemoglobinopathy treatment effectiveness prediction method
A technology for hemoglobinopathy and fetal hemoglobin, which is applied in blood diseases, gene therapy, genetic active ingredients, etc., can solve the problems of complex and harmful fetal hemoglobin expression mechanism, and achieve the effect of reducing the risk of failure or weakening of effectiveness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0270] 1. Use of modified TR cells for the preparation of a medicament for use in a method of treating a hemoglobinopathy in an individual, said method comprising:
[0271] a) An assessment step comprising assessing the ability of a first population of modified CD34-positive hematopoietic stem / progenitor cells ("CD34-positive HSPCs") to produce desired levels of gamma-globulin or fetal hemoglobin (HbF) following differentiation , wherein the first population of modified CD34-positive HSPCs is derived from said individual and modified to reduce BCL11A function ("modified EV cells"); and
[0272] b) a treatment step comprising administering to said individual a second population of modified CD34-positive HSPCs derived from said individual and modified to reduce BCL11A function ("modified TR cells ").
[0273] 2. Use of modified TR cells for the manufacture of a medicament for use in a method of treating a hemoglobinopathy in an individual, said method comprising a treatment ste...
Embodiment 1
[0322] Example 1: Gene Editing of Hematopoietic Stem Cell BCL11A Enhancer
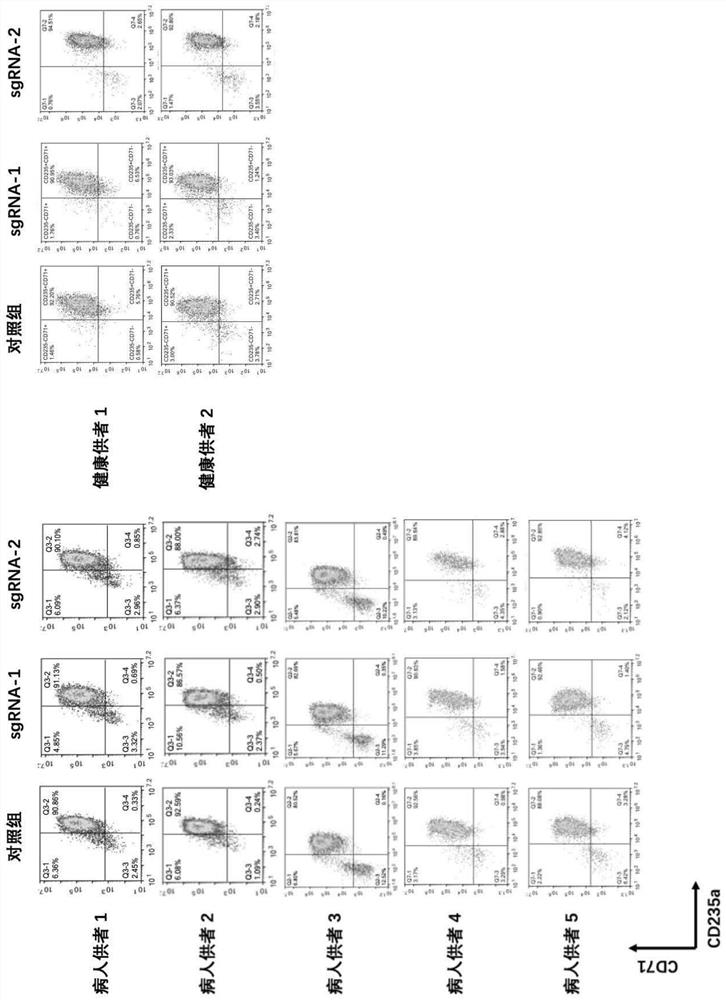
[0323] This example involves using the CRISPR / Cas9 system to gene-edit thalassemia (hereinafter referred to as thalassemia) patients and healthy donors to mobilize the BCL11A erythroid enhancer site of CD34-positive hematopoietic stem cells derived from peripheral blood.
[0324]Using the "CRISPR RGEN TOOLS" software to design sgRNA targeting the BCL11A (+58) site and synthesize two chemically modified sgRNAs, the sequence information is as follows: sgRNA-1: ctaacagttgcttttatcac (SEQ ID NO: 5), sgRNA-2: atcagaggccaaacccttcc SEQ ID NO:4. Cas9 mRNA编码序列信息如下:gacaagaagtacagcatcggcctggacatcggcaccaactctgtgggctgggccgtgatcaccgacgagtacaaggtgcccagcaagaaattcaaggtgctgggcaacaccgaccggcacagcatcaagaagaacctgatcggagccctgctgttcgacagcggcgaaacagccgaggccacccggctgaagagaaccgccagaagaagatacaccagacggaagaaccggatctgctatctgcaagagatcttcagcaacgagatggccaaggtggacgacagcttcttccacagactggaagagtccttcctggtggaagaggataagaagcacgagcggcaccccatcttc...
Embodiment 2
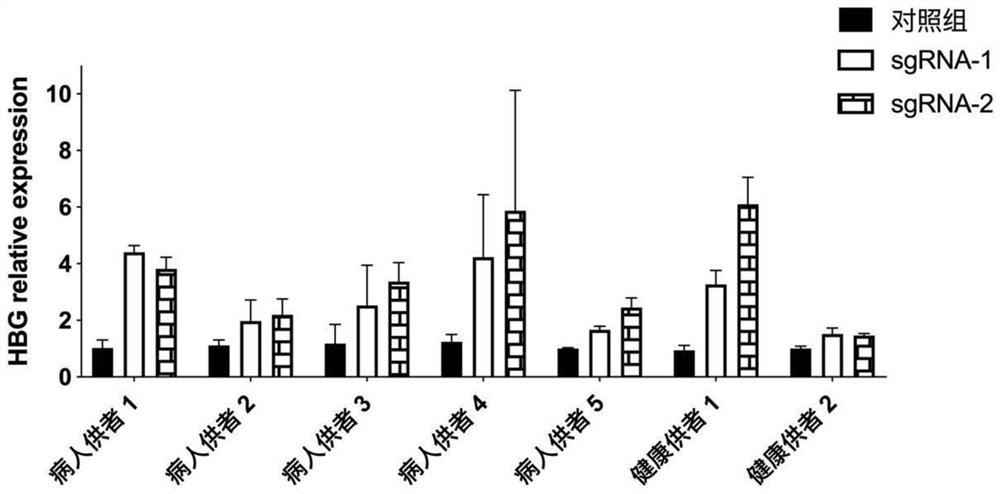
[0333] Example 2 Expression of γ-globin mRNA and erythroid differentiation-related proteins
[0334] This experiment verified the expression of γ-globin (HBG gene) mRNA after differentiation of gene-edited hematopoietic stem cells derived from peripheral blood mobilized from thalassemia patients and healthy donors.
[0335] 2.1 Red blood cell differentiation
[0336] The electroporation conditions of 300v 1ms were selected, and Cas9 mRNA and sgRNA-1, Cas9 mRNA and sgRNA-2 were respectively electroporated into 5 thalassemia patients and 2 healthy donors to mobilize hematopoietic stem cells derived from peripheral blood. Cells in the control group had no electroporation step. Afterwards, erythroid differentiation experiments were performed using the "two-step" differentiation protocol described below.
[0337] The first step in the "two-step method" differentiation is to use the hematopoietic stem cell erythroid expansion and differentiation medium to induce the differentiatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com