Antibacterial application of vitamin D and composition thereof
A technology of vitamins and compositions, applied in the field of vitamin D compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
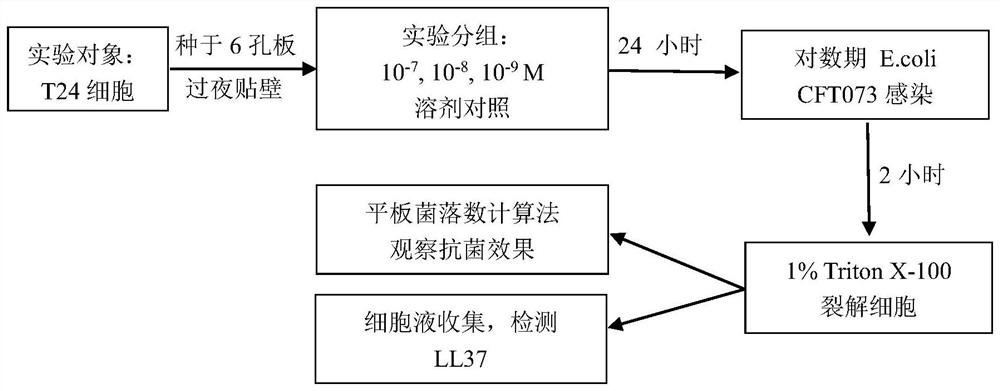
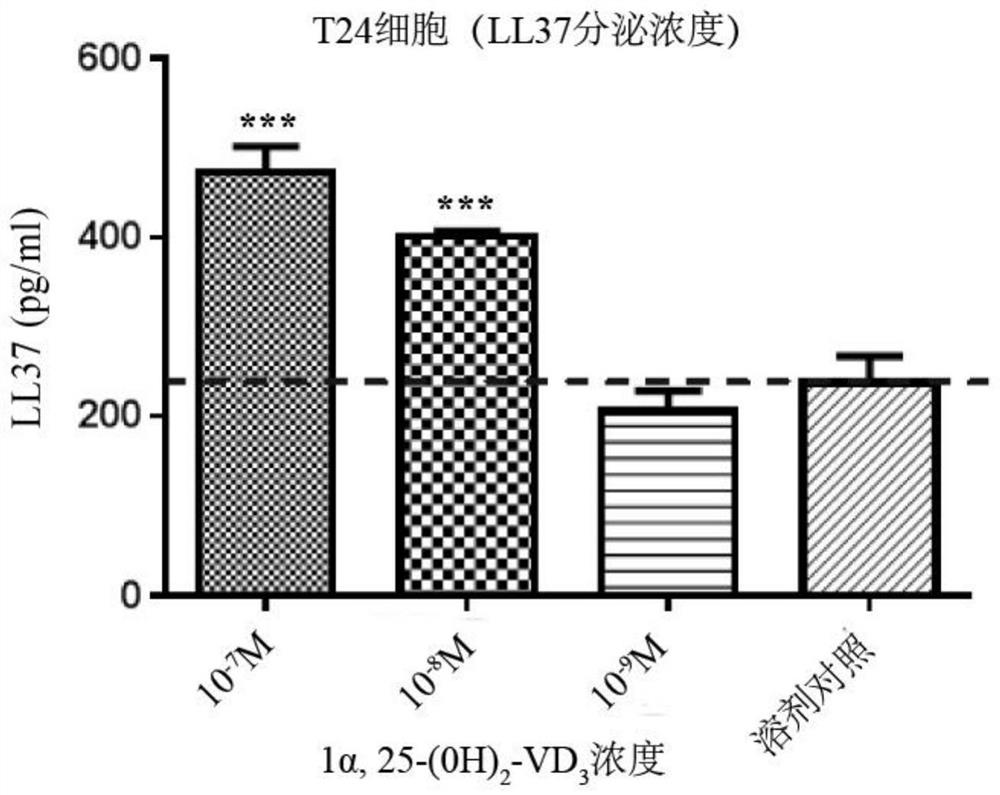
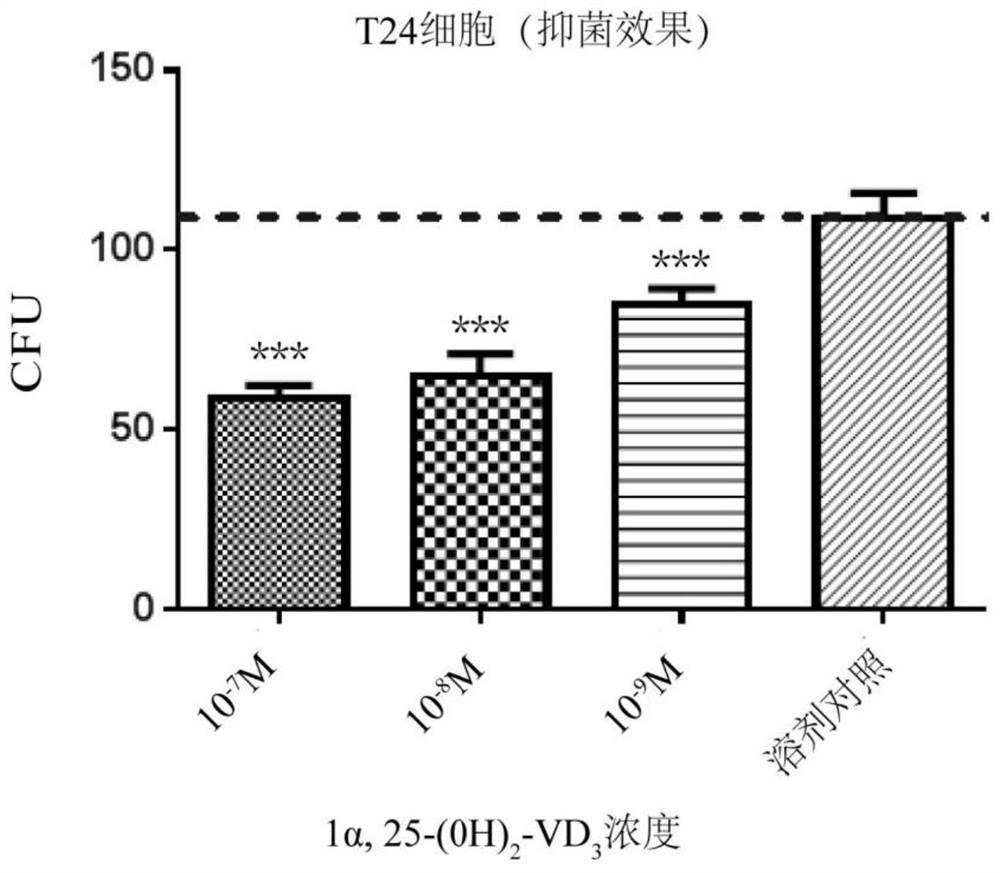
[0040] Example 1: 1α,25-(OH)2D3 (active form of vitamin D3) induces infection of E. coli T24 cells
[0041] Concentration detection of secreted LL37 antimicrobial peptide
[0042] 1 Purpose of the study
[0043] The purpose of this study was to investigate active vitamin D3 (1α,25-(OH) 2 D 3 ) whether pretreatment of urothelial cells can promote the cells to produce enough antimicrobial peptide LL37 to inhibit the infection of pathogenic E. 3 To provide a pharmacodynamic experimental basis for the treatment of recurrent lower urinary tract infections, and to clarify the quantitative relationship between the dose of vitamin D3 and the local secretion of antimicrobial peptides by the bladder epithelium.
[0044] 2 Experimental materials
[0045] 2.1 Test article
[0046] 1α,25-(OH) 2 D 3 (Vitamin D 3 active form)
[0047] Manufacturer: Sigma-Aldrich Purity: ≥99% (HPLC)
[0048] 2.2 Experimental cells and strains
[0049] 2.2.1 Experimental cells
[0050] T24 cells we...
Embodiment 2
[0092] Example 2: Evaluation of the in vitro antibacterial effect of antimicrobial peptide LL37 on common pathogenic bacteria isolated from clinical urinary tract in my country in the past three years
[0093] 1 Purpose of the study
[0094] To evaluate the in vitro antibacterial effect of antimicrobial peptide LL37 on common pathogenic bacteria isolated from clinical urinary tract in my country in the past three years.
[0095] 2 Test drugs
[0096] LL37: potency: 98.51%, Shanghai Zesheng Technology Development Co., Ltd.;
[0097] 3 Test strains
[0098] 3.1 Standard strain: Enterococcus faecalis ATCC29212, WHO14;
[0099] Enterococcus faecium ATCC700221;
[0100] Escherichia coli ATCC25922, ATCC35218.
[0101] 3.2 Clinical isolates: 76 clinical isolates from urinary tract were collected nationwide from 2015 to 2017, some of which were vancomycin-resistant Enterococcus faecium from 2013 and 2014, including:
[0102] 11 strains of vancomycin-sensitive Enterococcus faecal...
Embodiment 3
[0132] Example 3: Bacteriostatic ability of 1α,25-(OH)2D3 (active form of vitamin D3) on three cells (T24 cells, 5637 cells and SV-HUC-1 cells) infected with Escherichia coli
[0133] 1 Purpose of the study
[0134] The purpose of this study was to investigate active vitamin D3 (1α,25-(OH) 2 D 3 ) pretreatment of urothelial cells can inhibit the infection of the cells by pathogenic E. coli CFTO73, so that vitamin D 3 Pharmacodynamic experiments are provided for the treatment of recurrent lower urinary tract infections.
[0135] 2 Experimental materials
[0136] 2.1 Test article
[0137] 1α,25-(OH) 2 D 3 (Vitamin D 3 active form)
[0138] Manufacturer: Sigma-Aldrich Item No.: D1530 Purity: ≥99% (HPLC)
[0139] 2.2 Experimental cells and strains
[0140] 2.2.1 Experimental cells
[0141] 5637 cells and T24 cells were purchased from the cell bank of the Type Culture Collection Committee of the Chinese Academy of Sciences. The culture conditions were 1640 medium contai...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap