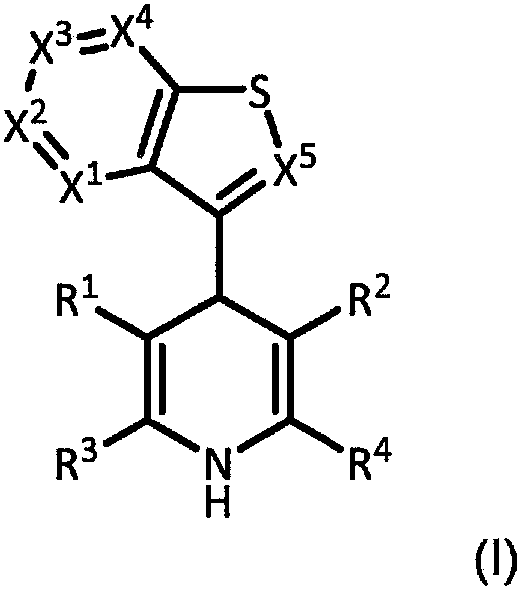
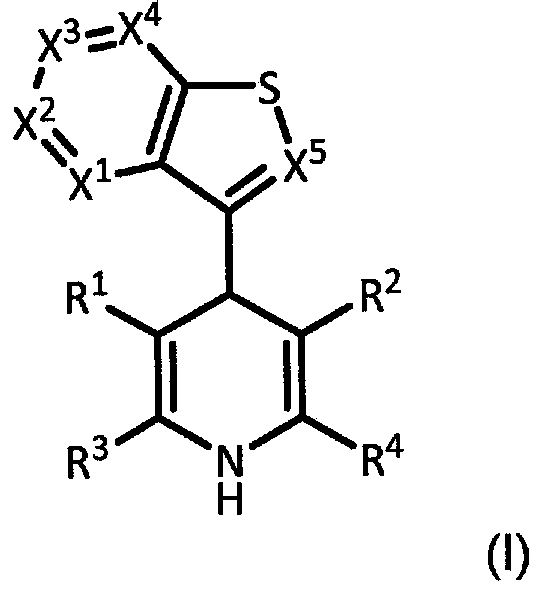
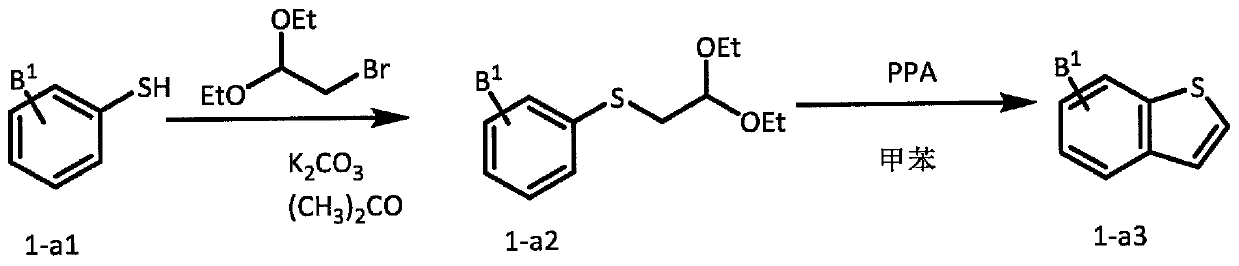
New analogs as androgen receptor and glucocorticoid receptor modulators
A compound, the technology of halogen atoms, applied in the field of dihydropyridine derivatives, can solve problems such as the treatment of cancer that has not been mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[1410] Preparation of dihydropyridine
[1411] Method A (Rampa, A et al., Forsch. 1992, 42, 1284).
Embodiment A1
[1412] Example A1: Methyl 5-acetyl-4-(benzo[b]thiophen-3-yl)-2,6-dimethyl-1,4-dihydro-pyridine-3-carboxylate
[1413]
[1414] 3-(Benzo[b]thiophen-3-ylmethylene)pentane-2,4-dione (0.24g, 0.98mmol, 1eq), methyl 3-oxobutyrate (0.158ml, 1.47mmol, 1.5eq) and 30% aqueous ammonium (0.62ml, 9.8mmol, 10eq) in i-PrOH (2ml) was heated for 12 hours. The solvent was removed under reduced pressure. The residue was taken up in water and extracted with dichloromethane and ethyl acetate. The combined organic layers were washed with brine, dried (Na 2 SO 4 ), filtered and concentrated, and used CH 2 Cl 2 The crude product was purified by flash column chromatography on silica gel with MeOH (1%) as eluent to give the title compound (114 mg, 25%) as a yellow solid.
[1415] 1 H-NMR (300MHz, CDCl 3 )δ=8.10(d, J=8.0Hz, 1H), 7.79(d, J=7.9Hz, 1H), 7.34(dt, J=24.0, 7.1Hz, 2H), 7.13(s, 1H), 6.27( bs, 1H), 5.47(s, 1H), 3.64(s, 3H), 2.34(s, 3H), 2.27(s, 3H), 2.14(s, 3H).
[1416] C 19 h 19...
Embodiment A4
[1418] Example A4: 5-Acetyl-4-(benzo[b]thiophen-3-yl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxamide
[1419] Method A yielded the title compound (73 mg, 22%) as a yellow solid.
[1420] 1 H-NMR (300MHz, CDCl 3 )δ=8.05(d, J=7.7Hz, 1H), 7.76(d, J=7.6Hz, 1H), 7.29(dt, J=15.0, 7.0Hz, 2H), 7.09(s, 1H), 6.76( s, 1H), 5.65 (bs, 2H), 5.34 (s, 1H), 2.25 (s, 3H), 2.08 (s, 6H).
[1421] C 18 h 18 N 2 o 2 HRMS (IE) m / z calculated for S: 326.0910, found: 326.1011.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com