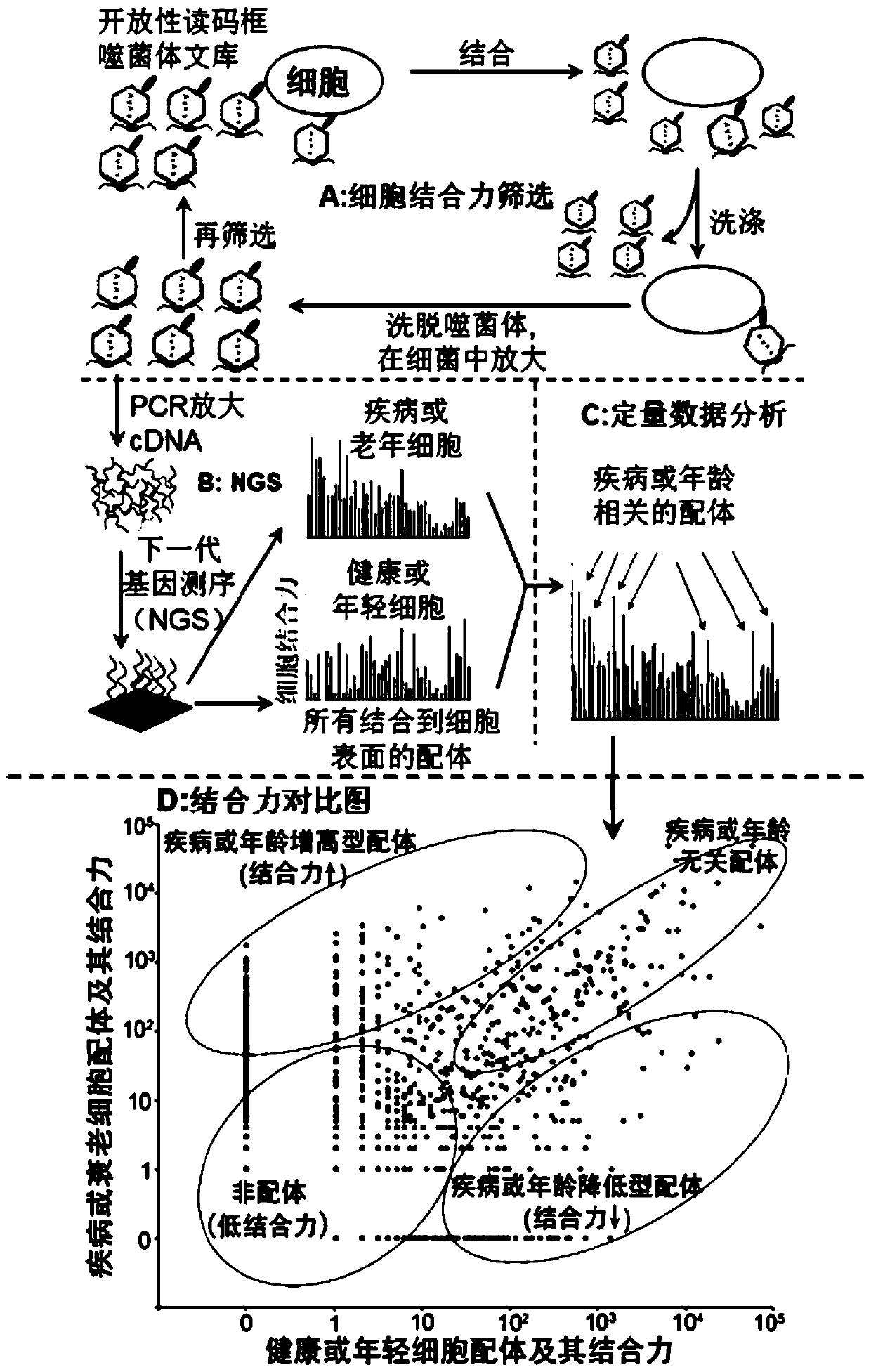
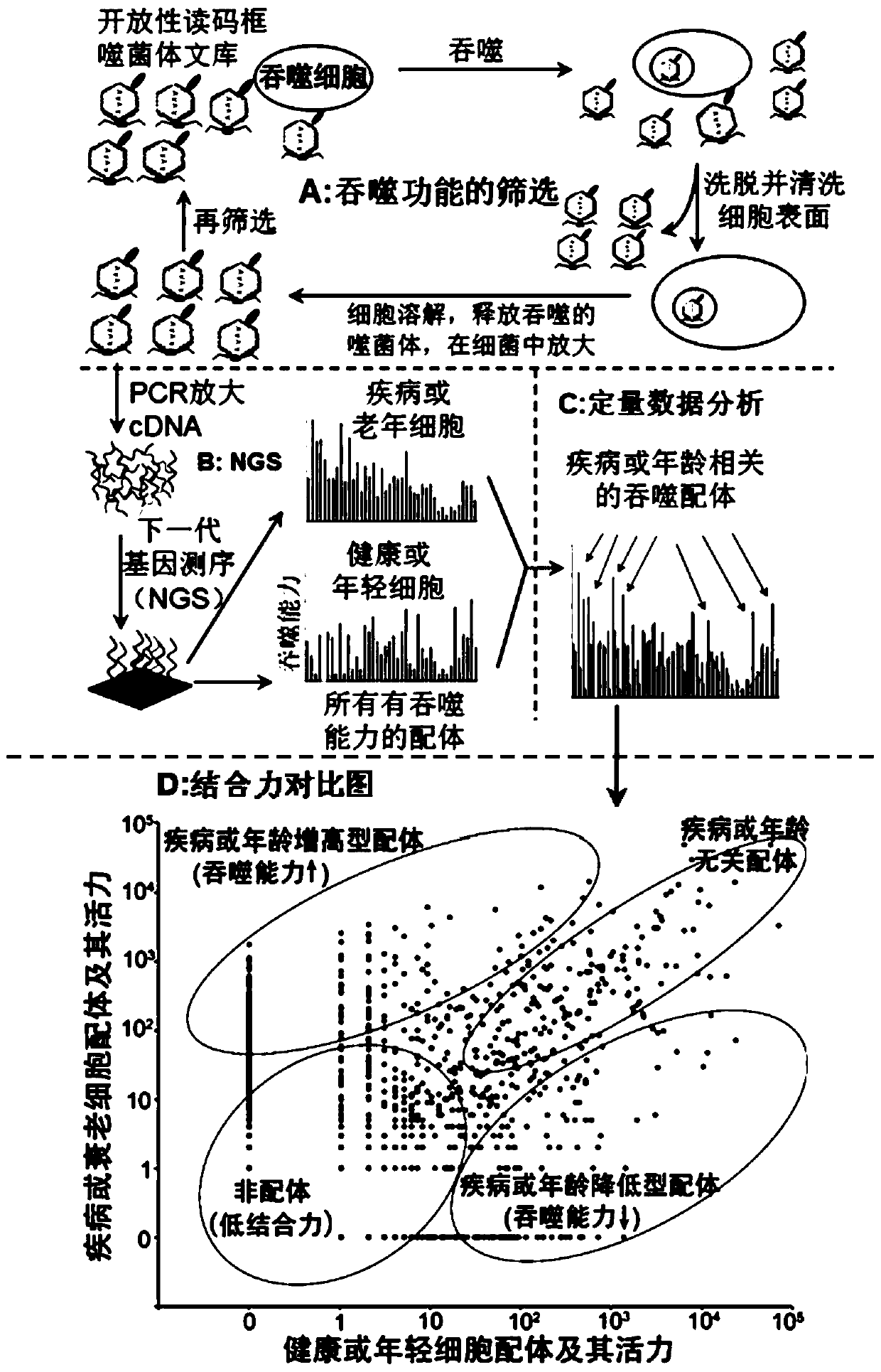
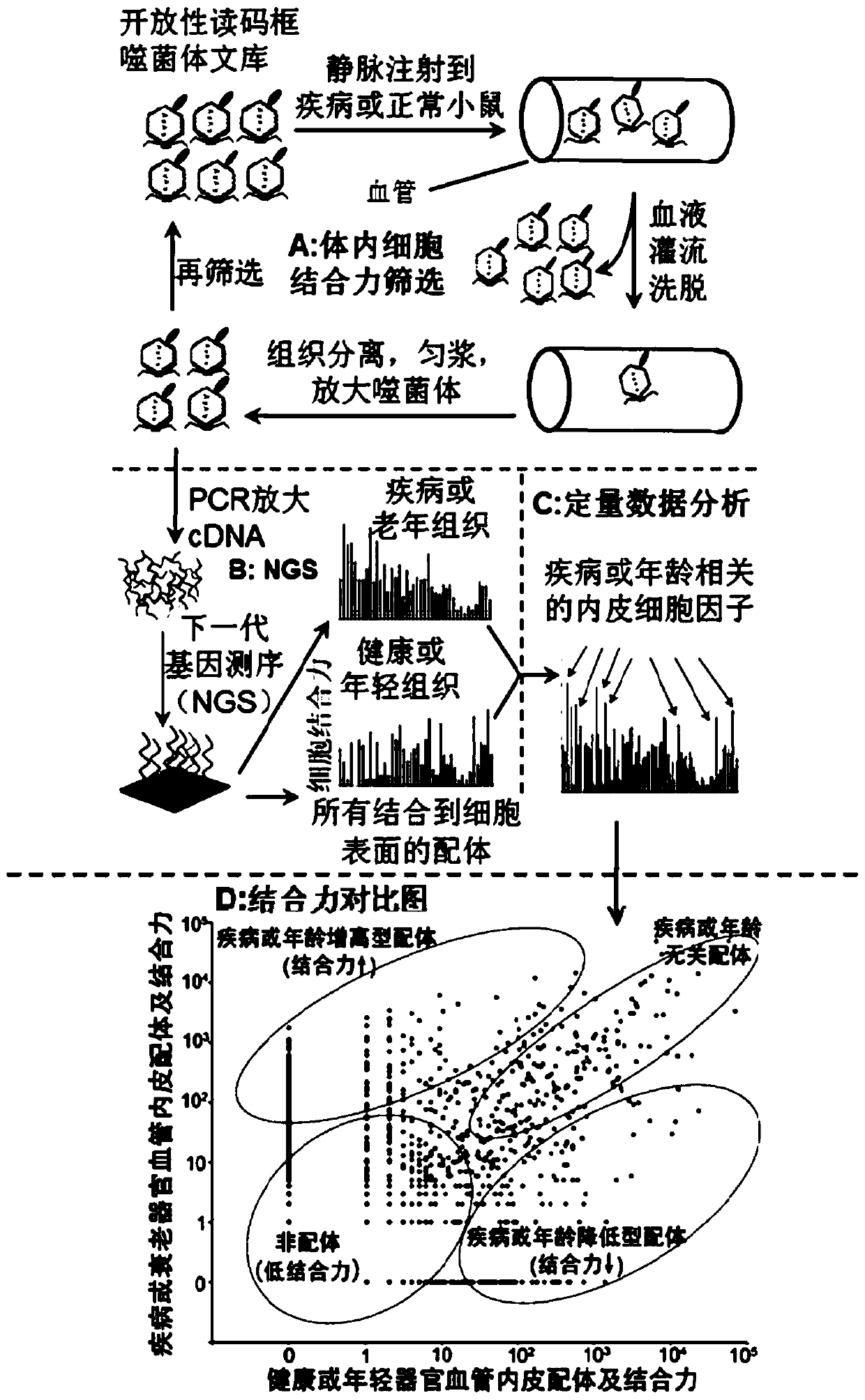
Application of Scg3 as a drug target in preparing drugs for treating vascular hyperplastic or exudative diseases
A technology for angiogenesis and ischemic diseases, applied in cardiovascular system diseases, metabolic diseases, bone diseases, etc., can solve problems such as limited understanding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Systematic identification of specific vascular endothelial ligands or factors for diabetic retinopathy
[0041] There are 378 million diabetic patients in the world, of which 93 million suffer from diabetic retinopathy (diabetic retinopathy, RD). Diabetic retinopathy is a major cause of blindness, including diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR). There are 21 million diabetic macular edema patients and 17 million proliferative diabetic retinopathy patients worldwide.
[0042] Diabetic retinopathy is characterized clinically by increased vascular permeability, endothelial cell death, increased acellular capillaries, leukostasis, advanced vascular proliferation, retinal hemorrhage, and vision loss. Ranibizumab (Lucentis, ranibizumab) was approved by the US FDA in 2012 as the first anti-angiogenic drug for the treatment of DME. Clinical trials have proven that Lucentis is effective in treating DME between 21% and 37%. The la...
Embodiment 2
[0068] Example 2 Systematic identification of tumor-specific vascular endothelium and factors
[0069] Another embodiment and application example of the present invention is to apply ligandomics technology to tumor mice and systematically identify tumor-specific vascular endothelial cells. and to directly identify tumor-associated vascular endothelial factors in vivo.
[0070] Many angiogenesis factors have been proved to promote angiogenesis in tumor growth and play a decisive role in tumor growth. Many angiogenic factors and angiostatic factors have been identified, and several have been approved by the US Food and Drug Administration for anti-angiogenic therapy of tumors. However, due to various technical difficulties, all known endothelial factors have been identified individually by various methods. Therefore, it is not known how many tumor-associated vascular endothelial ligands or factors need to be identified and used in cancer therapy. This difficulty also limits o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com