Production and application of fused antibacterial protein
A technology of fusion protein and recombinant bacteria, which can be used in antibacterial drugs, fusion peptides, peptide/protein components, etc., and can solve problems such as unstable lysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Synthesis of anti-staphylococcus fusion protein gene and construction of prokaryotic expression vector
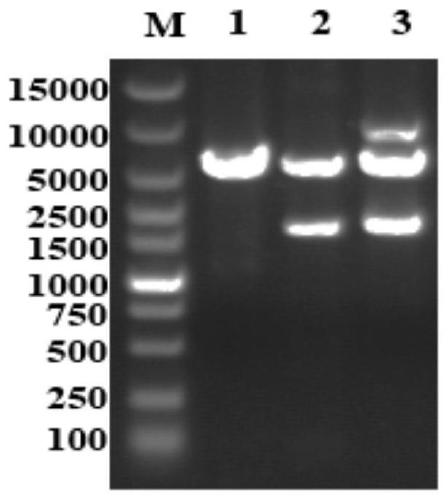
[0046] a. Download the full-length Staphylococcus aureus phage K genome (AY176327) from GenBank, intercept the nucleotide sequences at 27072-27762 and 28638-29435 bp, and obtain the full-length LysK gene. The amino acid sequence of the antimicrobial peptide Mersacidin was obtained from the APD database (The Antimicrobial Peptide Database, http: / / aps.unmc.edu / AP / ). The LysK gene is connected in series with the nucleotide sequence encoding the amino acid sequence of the antimicrobial peptide Mersacidin, and the nucleotide sequence is optimized using the codon usage preference of Escherichia coli, and it is named LysK-Mer, and its nucleotide sequence is as shown in SEQ ID NO: 1.
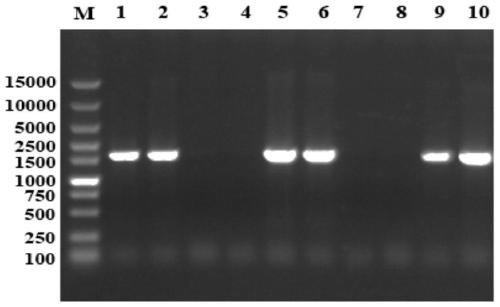
[0047] b. Add BamH I and Xho I restriction site sequences to both ends of the optimized anti-staphylococcal fusion protein gene and send it to Suzhou Jinweizhi Biotechnology Co., Lt...
Embodiment 2
[0049] Example 2 Induced expression and purification of anti-staphylococcal fusion protein
[0050] a. The recombinant plasmid pET32a-LysK-Mer obtained in Example 1 was transformed into Escherichia coli BL21 (DE3) competent according to conventional molecular biology methods, spread on a plate containing 50 μg / mL ampicillin and cultured overnight at 37°C, positive clones After amplification and shaking, the recombinant bacteria were obtained and named as BL21(pET32a-LysK-Mer).
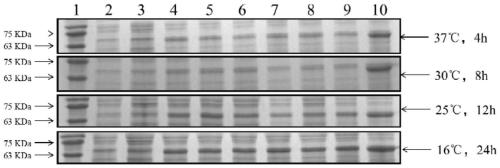
[0051] b. Transfer BL21 (pET32a-LysK-Mer) into 100mL LB medium, culture at 37°C, wait until OD 600 When it reached 0.5, IPTG was added at final concentrations of 0, 0.2, 0.4, 0.6, 0.8, and 1.0 mmol / L, respectively, and protein expression was induced at 4°C, 16°C, 25°C, and 37°C, respectively.
[0052] c. After the induction, the bacteria were collected, the cells were disrupted by ultrasonic waves, the supernatant was collected after centrifugation (12000 rpm), and the protein expression in the supern...
Embodiment 3
[0055] The plate antibacterial test of embodiment 3 anti-staphylococcal fusion proteins
[0056] Staphylococcus aureus (CICC 10001, CICC 10145, CICC 10201), Staphylococcus epidermidis (CICC10294), Staphylococcus pseudointermediate (CICC 10499), Escherichia coli (CICC 10389), Bacillus subtilis (CICC10275), fecal Enterococcus (CICC 23658), Salmonella enteritidis (CICC 21482) and Clostridium perfringens (CICC22949) were recovered, and 100 μL of fresh bacterial solution (bacteria content 109 CFU / mL) was evenly spread on LB solid medium. Take 100 μL of the anti-staphylococcal fusion protein obtained in Example 2 (adjust the concentration to 1 mg / mL), drop it in the center of the LB solid medium and spread it evenly, and add dropwise an equal amount of PBS buffer to dissolve the anti-staphylococcal fusion protein After cultured at 37°C for 12 hours, the lysing effect of the anti-staphylococcal fusion protein on different host bacteria was observed, and the results are shown in Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com