Therapeutic combination of a third-generation EGFR tyrosine kinase inhibitor and a raf inhibitor
A tyrosine kinase, EGFRT790M technology, applied in the field of treating cancer in human subjects, can solve the problem of incurable NSCLC
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
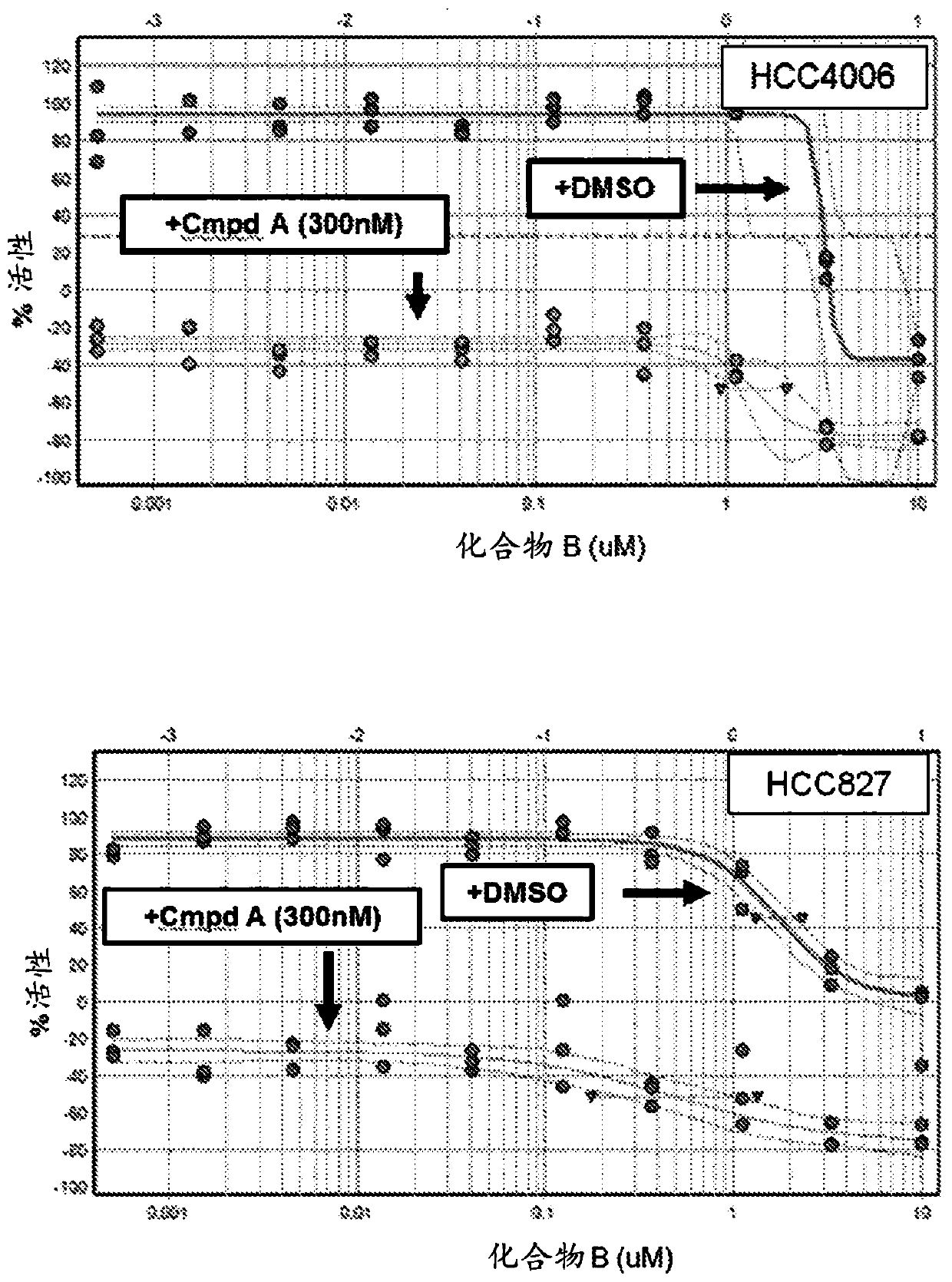
[0152] Example 1: Short term viability assay: Compound B enhances the efficacy of EGF816 (Compound A)
[0153] The potential efficacy of adding a MAPK pathway inhibitor (eg compound B) to a third generation EGFR tyrosine kinase inhibitor (eg EGF816) in EGFR mutant NSCLC was assessed below. A panel of EGFR mutant NSCLC cell lines were treated with a fixed dose (300 nM) of EGF816 ("Compound A") or DMSO in combination with Compound B over a range of 10 doses for 5 days.
[0154] method
[0155] cell line:
[0156] PC9, HCC827, HCC4006, NCI-H1975 and MGH707 are all EGFR mutant NSCLC cell lines sensitive to EGF816. PC9, HCC827, HCC4006 and NCI-H1975 were obtained from the Cancer Cell Line Encyclopedia (CCLE) database. MGH707 was obtained from Massachusetts General Hospital. All cell lines were maintained in RMPI medium supplemented with 10% fetal calf serum.
[0157] Compound:
[0158] Both Compound A (EGF816) and Compound B were resuspended in DMSO at a concentration of 10...
example 2
[0163] Example 2: Long-Term Viability Assays Show that Combinations of Third Generation EGFR Tyrosine Kinase Inhibitors and Raf Inhibitors Slows the growth of drug-resistant cells.
[0164] The combination of Compound A and Compound B was further tested in a long-term drug combination growth assay. The same EGFR mutant NSCLC cell line used in Example 1 above was treated with EGF816 alone or in combination with Compound B over a range of 5 doses for 14 days as follows.
[0165] method
[0166] PC9 (6000 / well), HCC827 (4000 / well), HCC4006 (5000 / well), and MGH707 (5000 / well) cells were placed in a 96-well plate, and treated with EGF816 (300nM)+DMSO or Compound B was treated for two weeks at a dose range of (0.03, 0.1, 0.3, 1 and 3uM). Drugs are updated twice a week. Cell confluence was used as a surrogate indicator of cell number and was measured by incucyte zoom at t=0, 4, 7, 10 and 14 days of treatment.
[0167] result
[0168] As can be seen from Figure 2, compound B ...
example 3
[0171] Example 3: Phase Ib, Open Label EGF816 in Combination with Compound B in Patients with EGFR Mutant NSCLC Signature, dose escalation and / or dose expansion studies.
[0172] Patients eligible for this study were those with advanced EGFR-mutant NSCLC, a disease currently incurable by any therapy. In first-line, treatment-naïve patients, or patients with acquired EGFR T790M gatekeeper mutations, and / or patients not previously treated with third-generation EGFR TKIs, treatment with EGF816 (Compound A) as a single agent is expected to be effective for most patients with clinical benefit. However, all patients are expected to develop treatment resistance and eventual disease progression after a period of single-agent EGF816 treatment.
[0173] In the context of EGF816 treatment, Compound B is expected to be active in tumors where signaling from or upstream from BRAF, including activated RTK and Ras signaling, drives resistance or tumor cell persistence. As shown above, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com