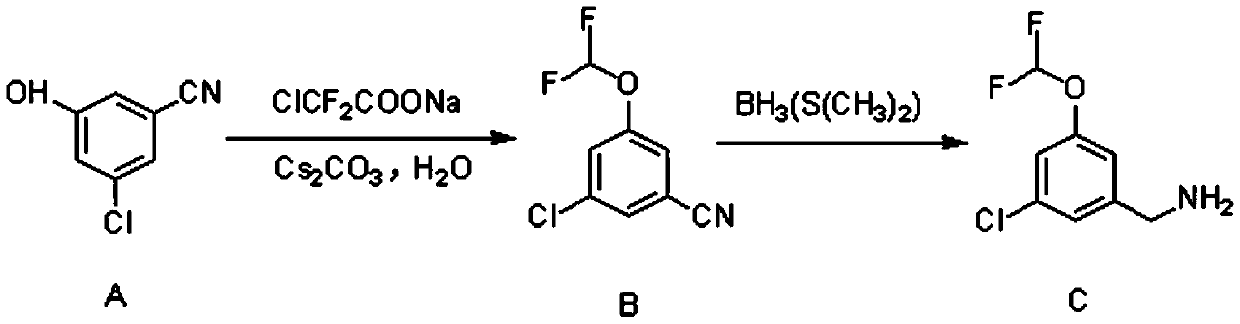
Synthetic method of 3-chloro-5-(difluoromethoxy)benzylamine
A technology of difluoromethoxy and synthetic methods, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as no synthetic methods reported in literature, and achieve the effects of easy control, reasonable process design, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 3-Chloro-5-hydroxybenzonitrile (23.5g, 150mmol, 1eq.) was dissolved in 450ml N,N-dimethylformamide, and sodium difluorochloroacetate (57.2g, 375mmol, 2.5eq. ), cesium carbonate (74.1g, 225mmol, 1.5eq.) and 45g of water, under the protection of nitrogen, the temperature was raised to 100°C, and the reaction was kept for 5 hours.
[0021] After the reaction was finished, 900ml of water was added to the reaction solution and stirred. Extract with ethyl acetate, separate the layers, and concentrate the organic phase. After purification by column chromatography, 28.4 g of yellow oily substance 3-chloro-5-(difluoromethoxy)benzonitrile was obtained, with a yield of 93%.
[0022] 3-Chloro-5-(difluoromethoxy)benzonitrile (28.4g, 139.5mmol, 1eq.) was dissolved in 300ml of anhydrous tetrahydrofuran and cooled to 0°C. Under nitrogen protection, borane dimethyl sulfide (41.9ml, 418.5mmol, 3eq.) was added dropwise to the above reaction system, the temperature was raised to 65°C, an...
Embodiment 2
[0026] 3-Chloro-5-hydroxybenzonitrile (18.8g, 120mmol, 1eq.) was dissolved in 400ml N,N-dimethylacetamide, and sodium difluorochloroacetate (36.6g, 240mmol, 2eq.) was added successively , potassium carbonate (33.5g, 240mmol, 2eq.) and 35g of water, under the protection of nitrogen, the temperature was raised to 80°C, and the reaction was kept for 10 hours.
[0027] After the reaction was finished, 800ml of water was added to the reaction solution and stirred. Extract with ethyl acetate, separate the layers, and concentrate the organic phase. After purification by column chromatography, 20.5 g of yellow oil 3-chloro-5-(difluoromethoxy)benzonitrile was obtained, with a yield of 84%.
[0028] 3-Chloro-5-(difluoromethoxy)benzonitrile (20.5 g, 100.8 mmol, 1 eq.) was dissolved in 200 ml of anhydrous tetrahydrofuran and cooled to 0°C. Under nitrogen protection, borane dimethyl sulfide (30.2ml, 302.4mmol, 3eq.) was added dropwise to the above reaction system, the temperature was rai...
Embodiment 3
[0032] 3-Chloro-5-hydroxybenzonitrile (28.2g, 180mmol, 1eq.) was dissolved in 600ml of dimethyl sulfoxide, and sodium difluorochloroacetate (41.2g, 270mmol, 1.5eq.) and sodium carbonate ( 19.3g, 180mmol, 1eq.) and 55g of water, under the protection of nitrogen, the temperature was raised to 148°C, and the reaction was kept for 1 hour.
[0033] After the reaction was finished, 1200ml of water was added to the reaction solution and stirred. Extract with ethyl acetate, separate the layers, and concentrate the organic phase. After purification by column chromatography, 26.8 g of yellow oil 3-chloro-5-(difluoromethoxy)benzonitrile was obtained, with a yield of 73%.
[0034] 3-Chloro-5-(difluoromethoxy)benzonitrile (26.8g, 131.4mmol, 1eq.) was dissolved in 300ml of anhydrous tetrahydrofuran and cooled to 0°C. Under nitrogen protection, borane dimethyl sulfide (39.4ml, 394.2mmol, 3eq.) was added dropwise to the above reaction system, the temperature was raised to 30°C, and the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com