Usage of Roxadustat for treating sepsis
A technology for roxadustat and sepsis, applied in the field of new drug use, which can solve problems such as high fatality rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Roxadustat improves the survival rate of septic mice
[0017] 1 Experimental materials
[0018] The C57BL / 6j mice used in the present invention were purchased from Jiangsu Jicui Yaokang Biotechnology Co., Ltd. The Roxadustat used in the present invention was purchased from Selleck Company, and the lipopolysaccharide was purchased from Sigma-Aldrich Company.
[0019] 2 Experimental methods
[0020] 2.1 Mice administration method
[0021] 40 male C57BL / 6j mice (8 weeks old, body weight 22-25g) were fed in an SPF grade animal room, and were randomly divided into control group (n=10) and roxadustat group (n=10) after adaptive feeding for 1 week. 10), lipopolysaccharide group (n=10) and roxadustat+lipopolysaccharide group (n=10).
[0022] Experiment 1: Roxadustat (10 mg / kg / day) was intraperitoneally injected into mice in roxadustat group and roxadustat+lipopolysaccharide group, and PBS was injected intraperitoneally into mice in control group and lipopolysaccha...
Embodiment 2
[0026] Example 2 Roxadustat alleviates the reduction of blood pressure and body temperature in mice caused by sepsis
[0027] 1 Experimental materials
[0028] The C57BL / 6j mice used in the present invention were purchased from Jiangsu Jicui Yaokang Biotechnology Co., Ltd. The Roxadustat used in the present invention was purchased from Selleck Company, and the lipopolysaccharide was purchased from Sigma-Aldrich Company.
[0029] 2.1 Mice administration method
[0030] Seventeen male C57BL / 6j mice (8 weeks old, weighing 22-25g) were housed in an SPF animal room, and after one week of adaptive feeding, they were randomly divided into LPS group (n=9) and roxadustat + LPS group Group (n=8). The mice in the roxadustat+lipopolysaccharide group were intraperitoneally injected with roxadustat (10 mg / kg / day), and the mice in the lipopolysaccharide group were injected with PBS intraperitoneally. Two days later, the lipopolysaccharide group and the roxadustat+lipopolysaccharide group...
Embodiment 3
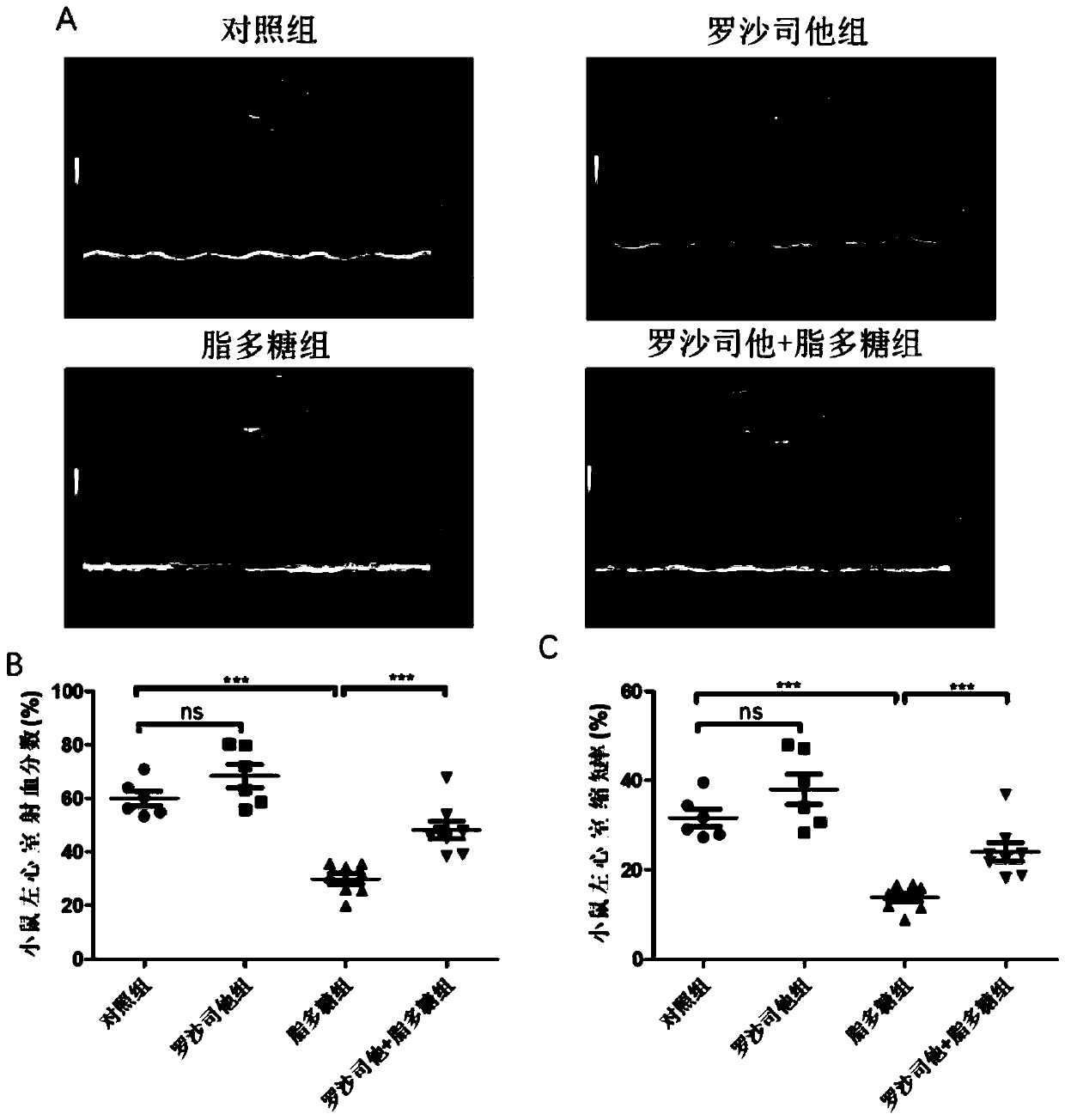
[0037] Example 3 Roxadustat relieves sepsis leading to decreased heart function in mice
[0038] 1 Experimental materials
[0039] The C57BL / 6j mice used in the present invention were purchased from Jiangsu Jicui Yaokang Biotechnology Co., Ltd. The Roxadustat used in the present invention was purchased from Selleck Company, and the lipopolysaccharide was purchased from Sigma-Aldrich Company.
[0040] 2 Experimental methods
[0041] 2.1 Mice administration method
[0042] Twenty-eight male C57BL / 6j mice (8 weeks old, weighing 22-25g) were fed in an SPF grade animal room, and were randomly divided into control group (n=6) and roxadustat group (n=6) after adaptive feeding for 1 week. 6), lipopolysaccharide group (n=8) and roxadustat+lipopolysaccharide group (n=8). The mice in the roxadustat group and the roxadustat+lipopolysaccharide group were intraperitoneally injected with roxadustat (10 mg / kg / day), and the mice in the control group and the lipopolysaccharide group were in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com