Manufacture technology for eye mask capable of alleviating dry eyes, eye irritation and asthenopia
A production process and visual fatigue technology, applied in the field of relieving dry eyes, the production process of eye patches for visual fatigue, and the field of eye astringency, can solve the problems of strong dependence, insignificant effects, and little progress in relieving visual fatigue. Small molecules, easy to dissolve in water, and relieve visual fatigue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
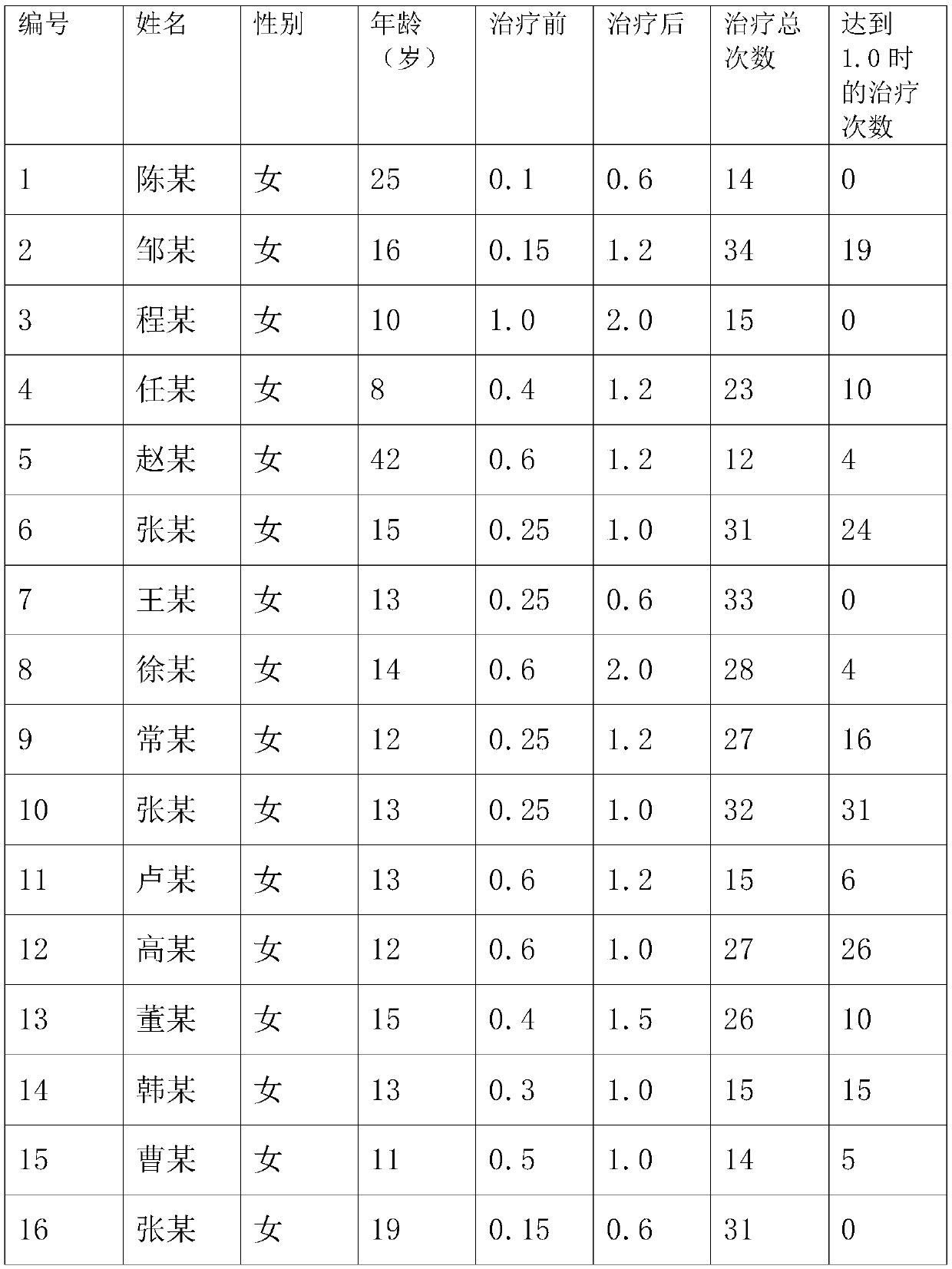
[0047] This embodiment discloses a manufacturing process of an eye patch for relieving dry eyes, eye astringency, and visual fatigue, which includes the following steps:
[0048] Step 1: take by weighing saffron, medlar, Achyranthes bidentata, Tribulus terrestris, Semen Cassiae, Semen Cassiae, Chrysanthemum Flos in a weight ratio of 1:1:1:1:1:1, and dry the above-mentioned medicines;
[0049] Step 2: Place the dried medicine in step 1 in a low-temperature storage at -80°C, carry out low-temperature wall breaking for 24 hours, take it out, and cool to normal temperature;
[0050] Step 3: put the cooled medicine in step 2 into distilled water, soak for 0.5-2h and then heat to 100°C;
[0051] Step 4: Take out the medicine in step 3, put it in the pressure change storage, and carry out vacuum wall breaking extraction to obtain the extract, the pressure is 4-11Mpa, and the vacuum degree is 0.08MPa;
[0052] Step five: the extract in step four is taken out, put into borneol to diss...
Embodiment 2
[0055] This embodiment discloses a manufacturing process of an eye patch for relieving dry eyes, eye astringency, and visual fatigue, which includes the following steps:
[0056] Step 1: take by weighing saffron, medlar, Achyranthes bidentata, Tribulus terrestris, Semen Cassiae, Semen Cassiae, Chrysanthemum Flos in a weight ratio of 1:1:1:1:1:1, and dry the above-mentioned medicines;
[0057] Step 2: Place the dried medicine in step 1 in a low-temperature storage at -80°C, carry out low-temperature wall breaking for 24 hours, take it out, and cool to normal temperature;
[0058] Step 3: put the cooled medicine in step 2 into distilled water, soak for 0.5-2h and then heat to 100°C;
[0059] Step 4: Take out the medicine in step 3, put it in the pressure change storage, and carry out vacuum wall breaking extraction to obtain the extract, the pressure is 4-11Mpa, and the vacuum degree is 0.15MPa;
[0060] Step 5: the extract in step 4 is taken out, put into borneol and dissolve,...
Embodiment 3
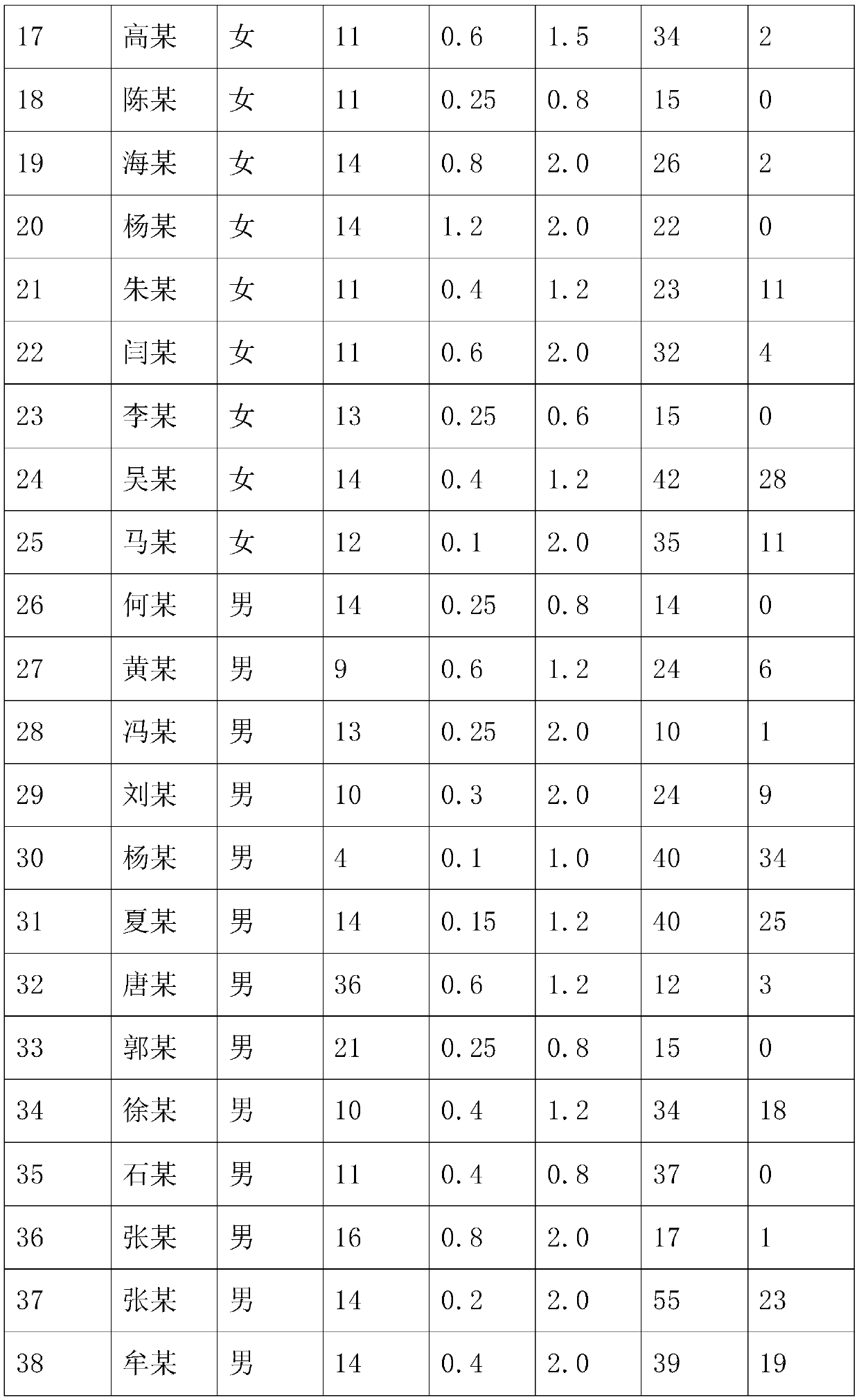
[0063] This embodiment discloses a manufacturing process of an eye patch for relieving dry eyes, eye astringency, and visual fatigue, which includes the following steps:
[0064] Step 1: take by weighing saffron, medlar, Achyranthes bidentata, Tribulus terrestris, Semen Cassiae, Semen Cassiae, Chrysanthemum Flos in a weight ratio of 1:1:1:1:1:1, and dry the above-mentioned medicines;
[0065] Step 2: Place the dried medicine in step 1 in a low-temperature storage at -80°C, carry out low-temperature wall breaking for 24 hours, take it out, and cool to normal temperature;
[0066] Step 3: put the cooled medicine in step 2 into distilled water, soak for 0.5-2h and then heat to 100°C;
[0067] Step 4: Take out the medicine in step 3, put it in the pressure change storage, and carry out vacuum wall breaking extraction to obtain the extract, the pressure is 4-11Mpa, and the vacuum degree is 0.12MPa;
[0068] Step 5: the extract in step 4 is taken out, put into borneol and dissolve,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com