Peptide vaccine and peptide vaccine composition for cranial nerve disease
A peptide vaccine and cranial nerve technology, applied in the field of peptide vaccines and peptide vaccine compositions, can solve the problems of malignant transformation, peripheral nerve injury, and multiple tumors that cannot be actively operated on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0105] In Embodiment 1, the present invention provides a method for treating cranial nerve diseases, the method comprising: administering an effective amount of a peptide that induces CTL against VEGFR-1 expressing cells, or inducing CTL against VEGFR-2 expressing cells, to a patient in need of treatment. CTL peptides.
[0106] In Embodiment 1, the present invention provides a peptide that induces CTL against VEGFR-1 expressing cells or a peptide that induces CTL against VEGFR-2 expressing cells for treating cranial nerve diseases.
[0107] In Embodiment 1, the present invention provides the use of a peptide that induces CTL against VEGFR-1 expressing cells or a peptide that induces CTL against VEGFR-2 expressing cells for the manufacture of a therapeutic drug for cranial nerve diseases.
[0108] In each of these embodiments, the peptides that induce CTLs against VEGFR-1 expressing cells and the peptides that induce CTLs against VEGFR-2 expressing cells include the above-menti...
experiment example 1
[0112] (Preparation of Peptide Vaccine Composition)
[0113] 2 mg of the peptide comprising the amino acid sequence described in SEQ ID NO: 1, 2 mg of the peptide comprising the amino acid sequence described in SEQ ID NO: 5, and 1 mL of incomplete Freund's adjuvant (model "MONTANIDEISA51", SEPPIC Corporation ) were mixed to prepare an emulsion to prepare a 2 mL peptide vaccine composition.
experiment example 2
[0115] (Administration of the peptide vaccine composition to the patient of case 1)
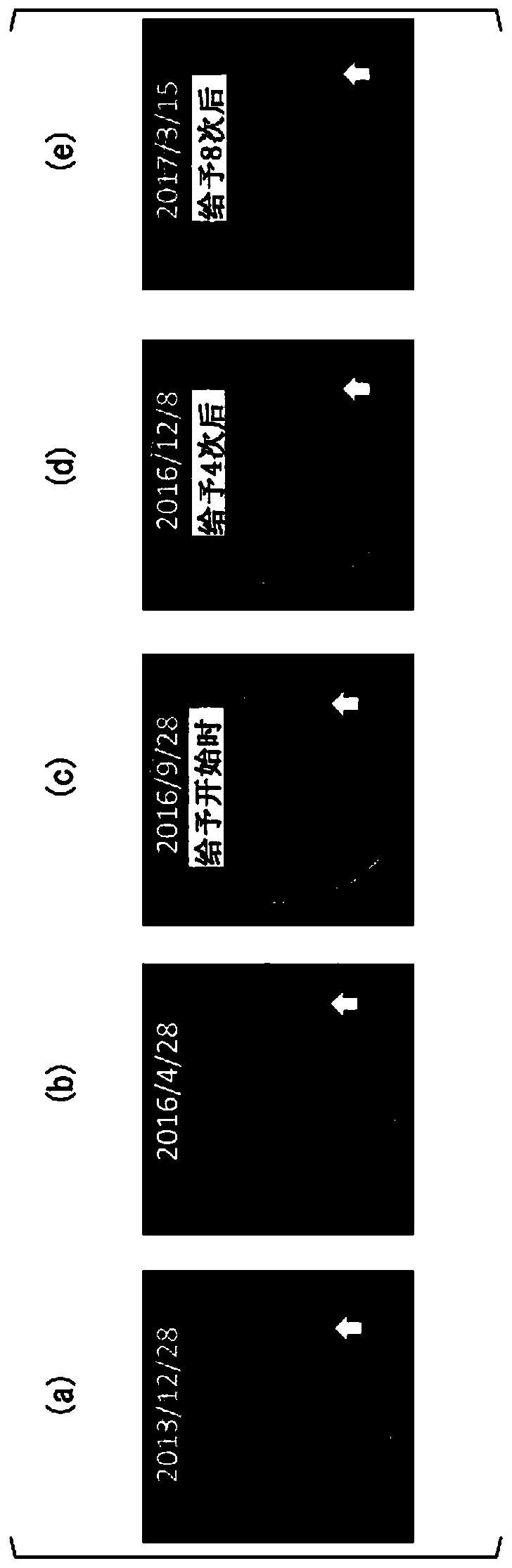
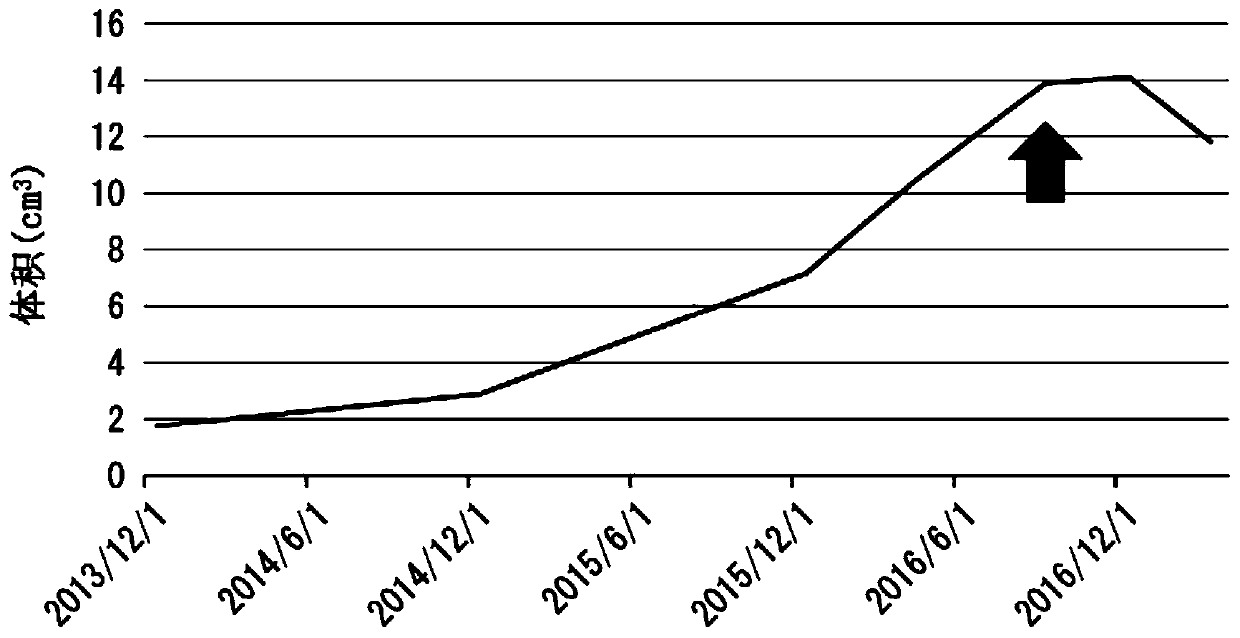
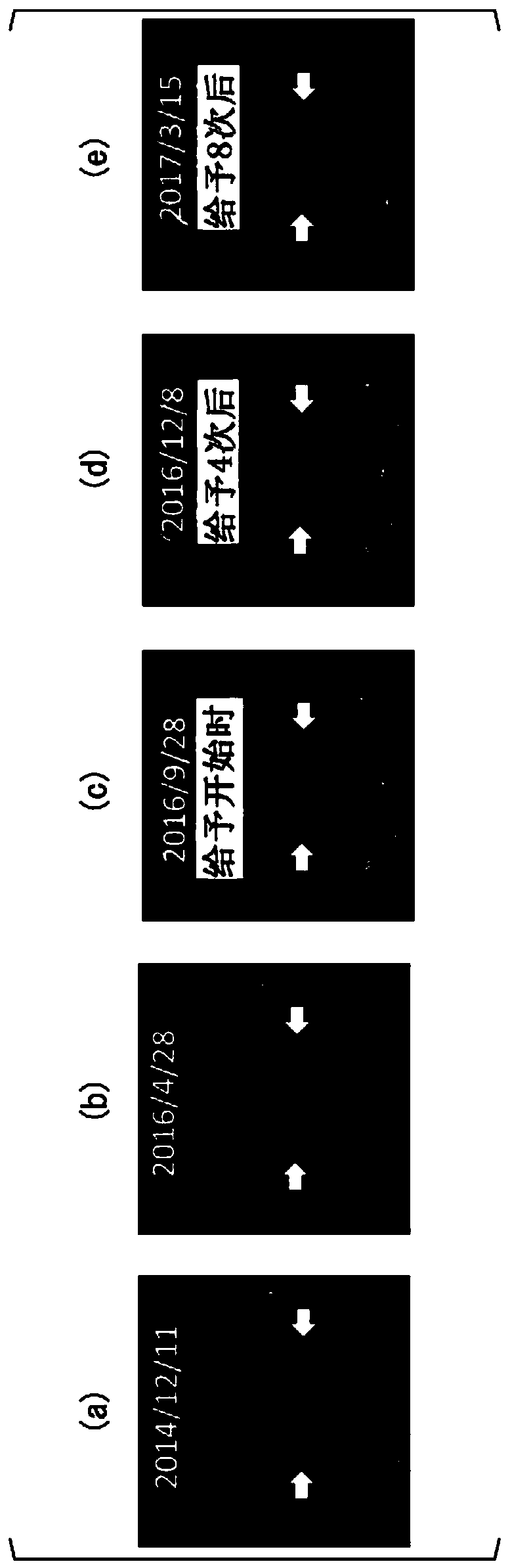
[0116] The patient of Case 1 was a 30-year-old male. The HLA type of the patient in Case 1 was HLA-A*24:02. Bilateral vestibular schwannomas, trigeminal schwannomas, and meningiomas develop intracranially, and numerous schwannomas in the spinal cord or skin. He has no hearing on both sides and is bedridden. A total of 4 tumor resections and 3 gamma-knife irradiations including intracranial or spinal cord were performed, but it was difficult to control the growth of the tumor, and the tumor grew year by year. The peptide vaccine composition prepared in the same manner as in Experimental Example 1 was administered to the patient of Case 1.
[0117] (give schedule)
[0118] 1 mL of the peptide vaccine composition was subcutaneously administered each time around the bilateral axillary or inguinal lymph nodes of the patient. As the initial administration, for one month from the start of the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com