Compositions and methods for cancer imaging and radiotherapy
A compound and chelate technology, which can be used in the detection, labeling, staging or treatment of cancer, cancers that overexpress low-density lipoprotein receptors, and can solve problems such as unmet medical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
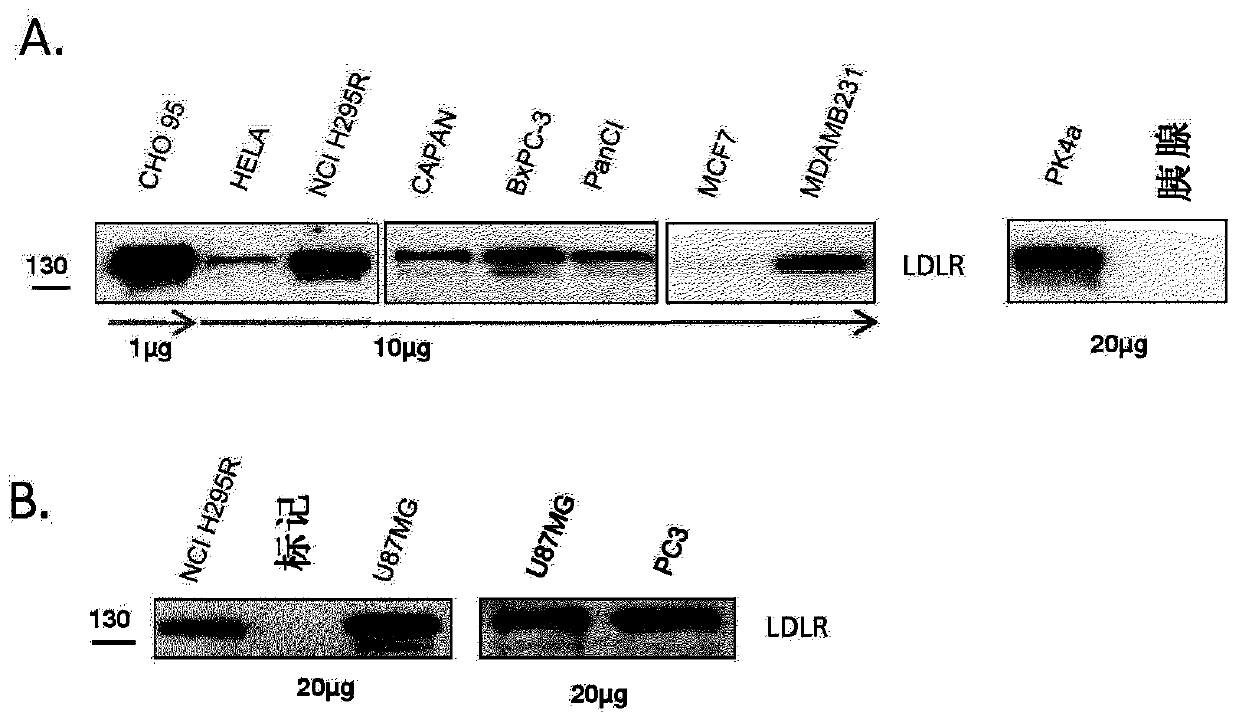
[0420] Example 1: LDL-receptor (LDLR) expression in mouse and human tissues
[0421] To assess membrane expression of LDLR in mouse and human cell lines of interest, membrane extracts were prepared from human and mouse cancer cell lines using the kit ProteoExtract Subcellular Proteome Extraction Kit (Calbiochem, La Jolla, CA, USA) things.
[0422] Membrane extracts were quantified using the BioRad DC protein assay (Bio-Rad, Hercules, CA, USA) following the manufacturer's instructions. 1, 10 μg or 20 μg of cell membrane proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on a 4-12% polyacrylamide gel and transferred to a nitrocellulose membrane (Amersham Biosciences) superior. The membrane was probed with a goat anti-LDLR antibody (R&D Systems, (1 / 500)) followed by a peroxidase-conjugated donkey anti-goat secondary antibody (Jackson Immunoresearch). Finally, the protein is detected using chemiluminescence.
[0423] Such as figu...
Embodiment 2
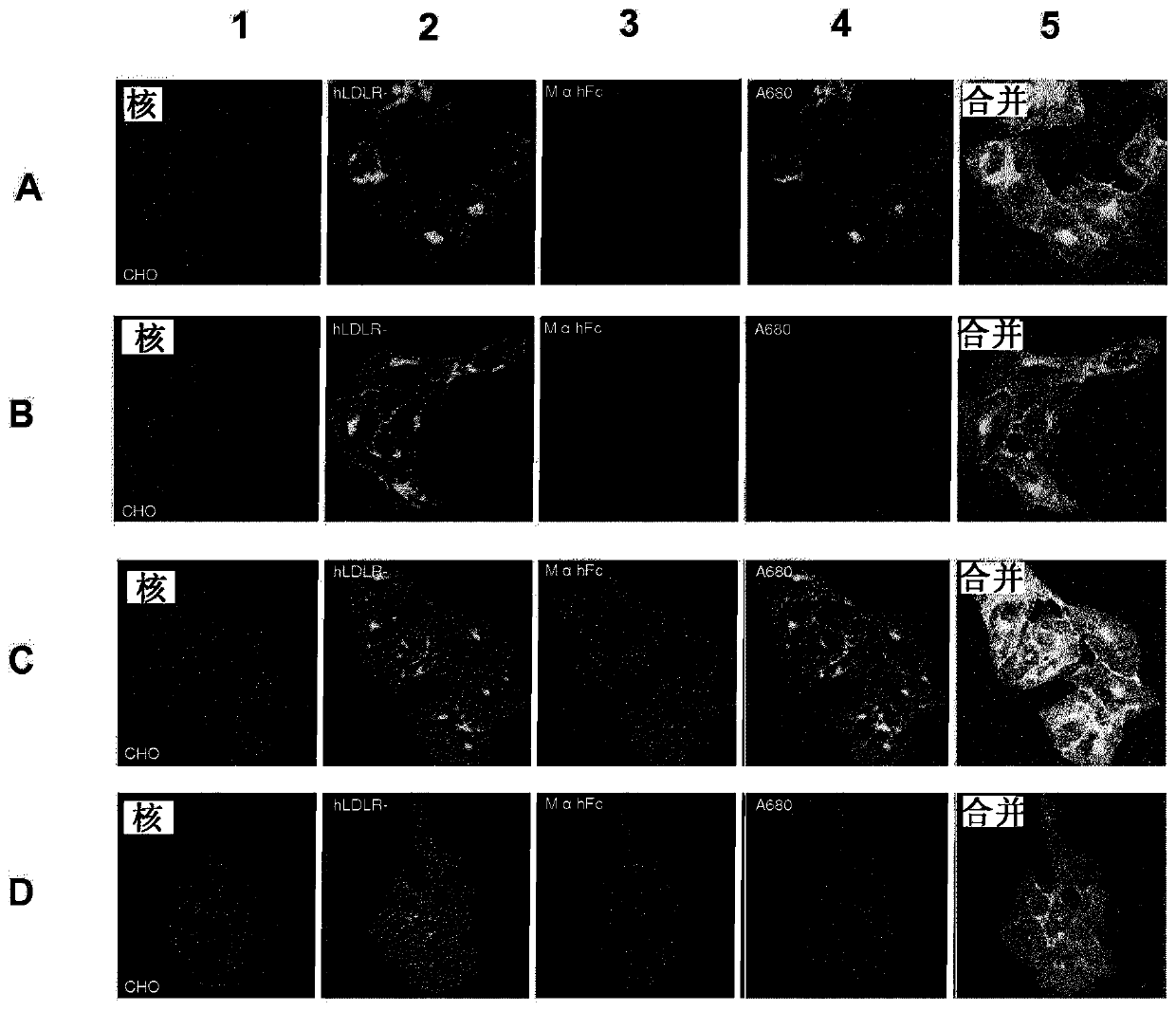
[0424] Example 2: Synthesis of fluorescent LDLR targeting conjugates: Conjugate A, Conjugate B, Conjugate C and Conjugate D
[0425] In the following examples, the production and use of conjugates A, B, C and D are disclosed. As shown below, conjugates A, B, C and D contain peptides A (SEQ ID NO: 1), B (SEQ ID NO: 13), C (SEQ ID NO: 6) and D (SEQ ID NO: 14).
[0426] compound composition Conjugate A Fc-peptide A-A680 Conjugate B Fc-peptide B-A680 Conjugate C Fc-peptide C-A680 Conjugate D Fc-peptide D-A680
[0427] Production of Conjugate C and Conjugate D Fusion Proteins
[0428] Peptides C and D were cloned as fusions to the Fc fragment of IgGl.
[0429] For the production of Fc fragments fused to peptides C or D, plasmid constructs were generated based on the plasmid pINFUSEhIgG1-Fc2 (InvivoGen) used as template. Use oligonucleotides to synthesize large primers called Primer C or Primer D by PCR:
[0430] - Forward primer:...
Embodiment 3
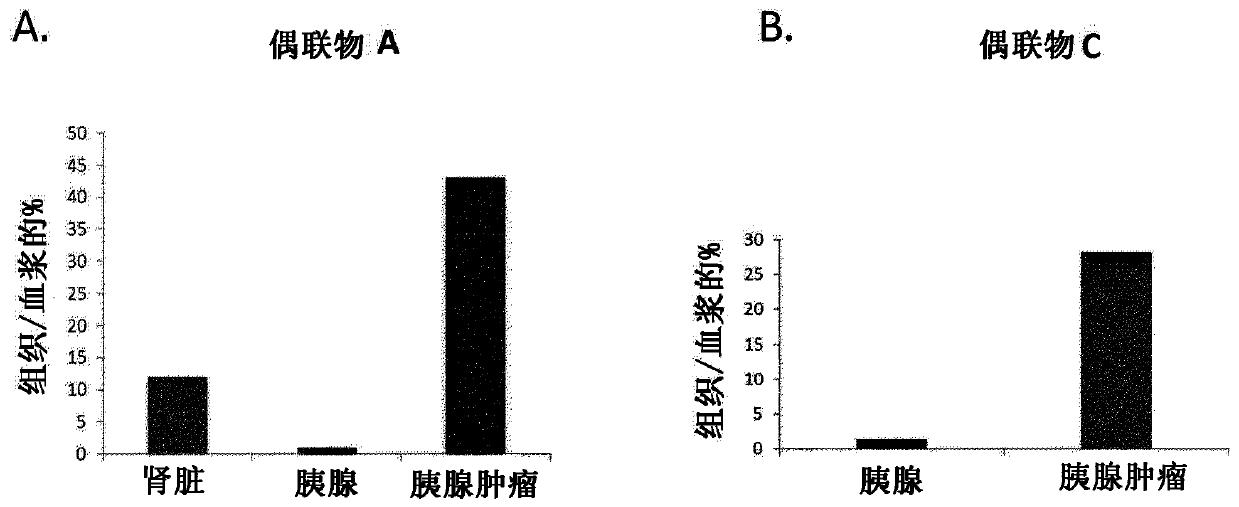
[0456] Example 3: Affinity of Conjugates A, B, C and D to LDLR (K D ) determined by surface plasmon resonance (SPR). Binding / endocytic properties of conjugates to h / mLDLR stably expressed by CHO cells and cancer cell lines.
[0457] The affinity of the conjugate to LDLR (K D )
[0458] Recombinant human LDLR (His-tagged) was purchased from Sino Biological (Beijing, China). Interaction of conjugates with LDLR was tested at 25°C using Biacore T200 (GE Healthcare) and 50 mM HEPES-NaOH pH 7.4, 150 mM NaCl, 0.005% Tween-20, 50 μM EDTA as running buffer. Add hLDLR at 35-60 fmol / mm 2 The densities of were immobilized on a NiHC sensor chip (Xantec, Dusseldorf, Germany). Binding of conjugates to LDLR-coated flow cells was corrected for non-specific binding to uncoated flow cells. A one-cycle kinetic approach was used to measure ligand affinity to LDLR. Ligands were diluted in running buffer and injected sequentially at 30 μl / min for 2 minutes using increasing concentrations...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com