Halofuginone with optical activity and synthesis method of intermediate of halofuginone
An optically active and chiral technology, applied in organic chemistry methods, organic chemistry, etc., can solve problems such as cumbersome synthetic routes, highly toxic use, and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
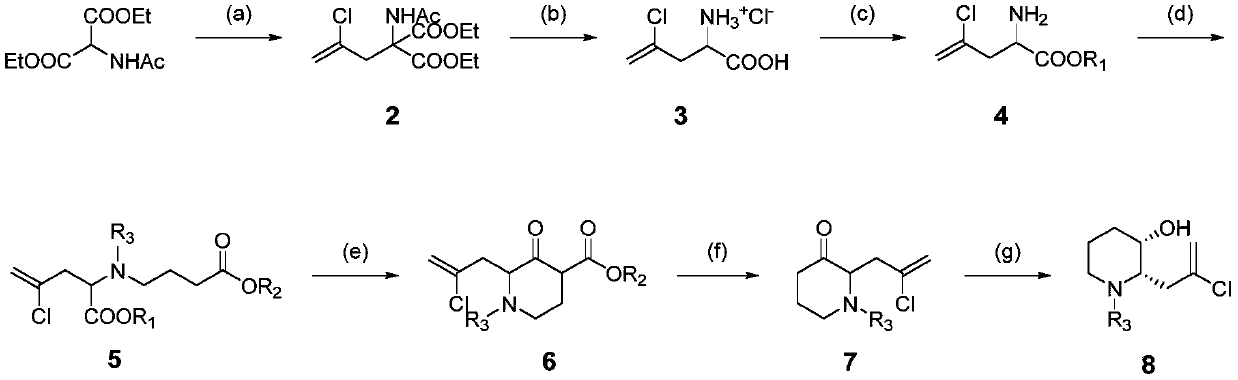
[0182] The synthesis of embodiment 1 racemic hemosanone
[0183] Intermediate compound 4 (R 1 = Synthesis of Et)
[0184]
[0185] Step (a): At room temperature, add diethyl acetamidomalonate (5.00kg, 23.02mol), anhydrous potassium carbonate (6.35kg, 46.04mol), potassium iodide (0.76kg, 4.6mol) into a 50L reactor , tetrabutylammonium bromide (0.37kg, 1.15mol) and acetonitrile (25L), after stirring for 20 minutes, 2,3-dichloropropene (3.07kg, 27.62mol) was added. The temperature was raised to 85-90°C for reaction, and the reaction was monitored by HPLC. After the reaction is finished, the temperature of the reaction liquid is lowered to within 25°C, and the diluted hydrochloric acid is slowly dropped into the reaction kettle to neutralize to pH 7-7.5. After standing still, the layers were separated, and the organic layer was concentrated under reduced pressure at 50°C. The concentrated residue was added with ethanol-water (1:10, 20 L) and stirred for 1 hour to crystalliz...
Embodiment 2
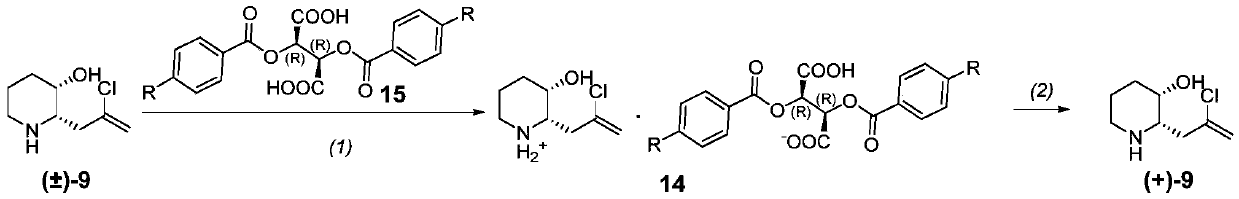
[0247] Synthesis of compounds of formula (+)-9 (dextrorotatory optically active [(+)-(2S,3S)-2-(2-chloropropenyl)-3-hydroxypiperidine])
[0248]
[0249]Step (1): At room temperature, add racemic compound of formula 9 (140 g, 0.797 mol, prepared according to the method of Example 1) and acetonitrile (1400 ml) into a 5 L three-neck flask, heat to 60 ° C and stir to dissolve. L-(-)-dibenzoyltartaric acid (300 g, 0.837 mol) of formula 15 was dissolved in acetonitrile (800 ml) and added dropwise to the reaction flask, and the drop was completed in about 10 minutes. After dropping, stir for 20-30 minutes, then move to room temperature and stir for 2 hours. Filter, rinse with acetonitrile (300ml), and drain to obtain the crude double salt. Add pure water-acetonitrile (1:4, 2000ml) to the crude product, stir, heat to 80°C to dissolve, filter while hot, and stir the filtrate at room temperature for 2 hours to crystallize. Filter, wash with acetonitrile, and dry to obtain the refi...
Embodiment 3
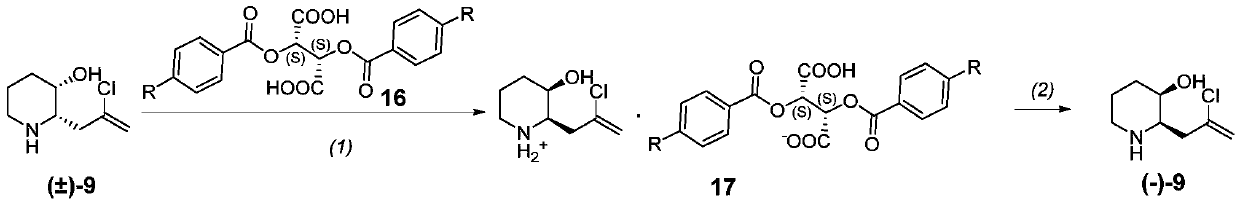
[0256] Synthesis of Compound of Formula (-)-9 (Levorotatory Optically Active [(-)-(2R,3R)-2-(2-Chloropropenyl)-3-Hydroxypiperidine])
[0257]
[0258] Step (1): At room temperature, add racemic compound of formula 9 (14 g, 79.7 mmol, prepared according to the method of Example 1) and acetonitrile (140 ml) into a 5 L three-neck flask, heat to 60° C. and stir to dissolve. L-(-)-dibenzoyltartaric acid (30g, 83.7mmol) of formula 16 was dissolved in acetonitrile (80ml) and added dropwise to the reaction flask, and the dropwise was completed in about 10 minutes. After dropping, stir for 20-30 minutes, then move to room temperature and stir for 2 hours. Filter, rinse with acetonitrile (30ml), and drain to obtain the crude double salt. Add pure water-acetonitrile (1:4, 200ml) to the crude product, stir, heat to 80°C to dissolve, then filter while hot, and stir the filtrate at room temperature for 2 hours to crystallize. Filter, wash with acetonitrile, and dry to obtain the refine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com