Preparation method of polymerase inhibitor used for tumor-related diseases
An inhibitor, polymerase technology, used in antitumor drugs, drug combinations, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
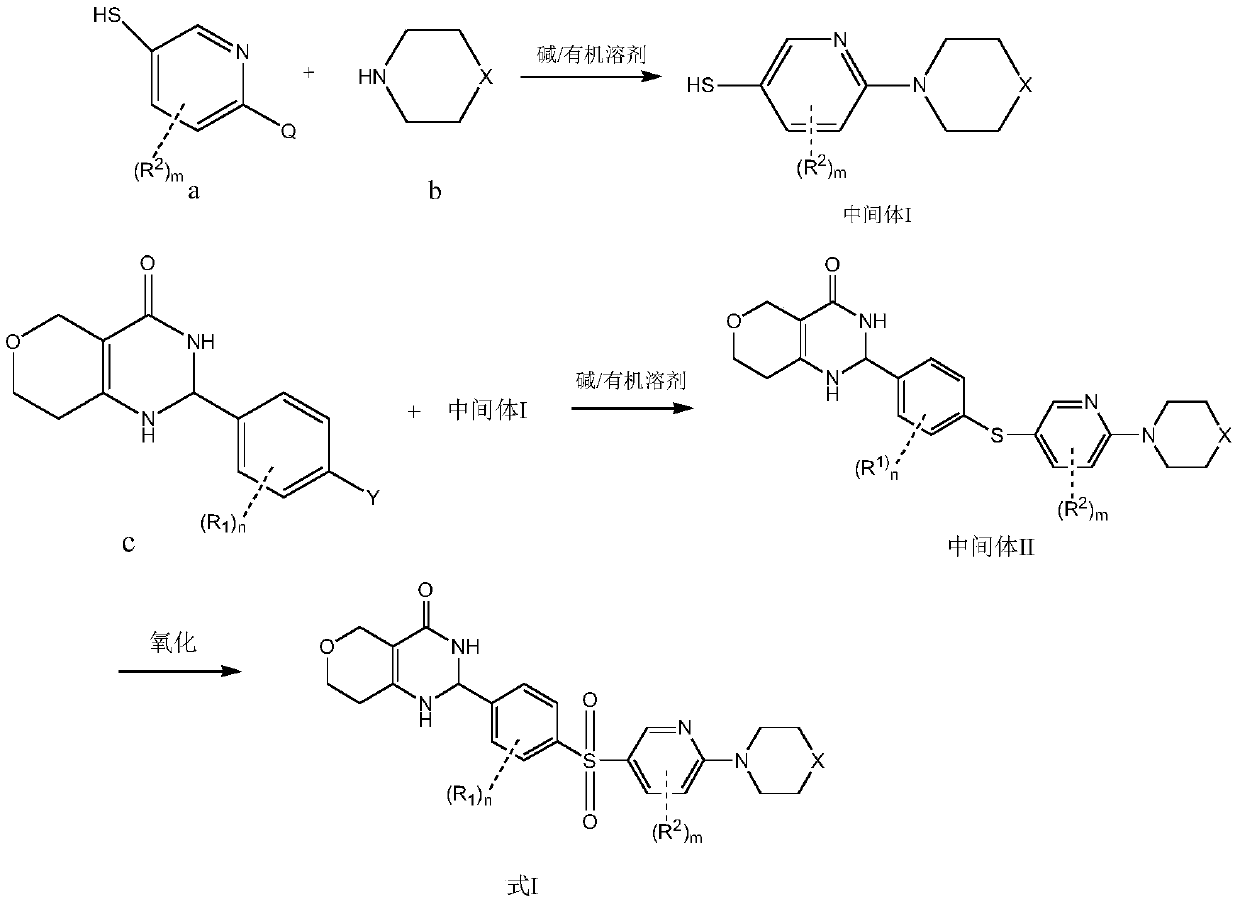
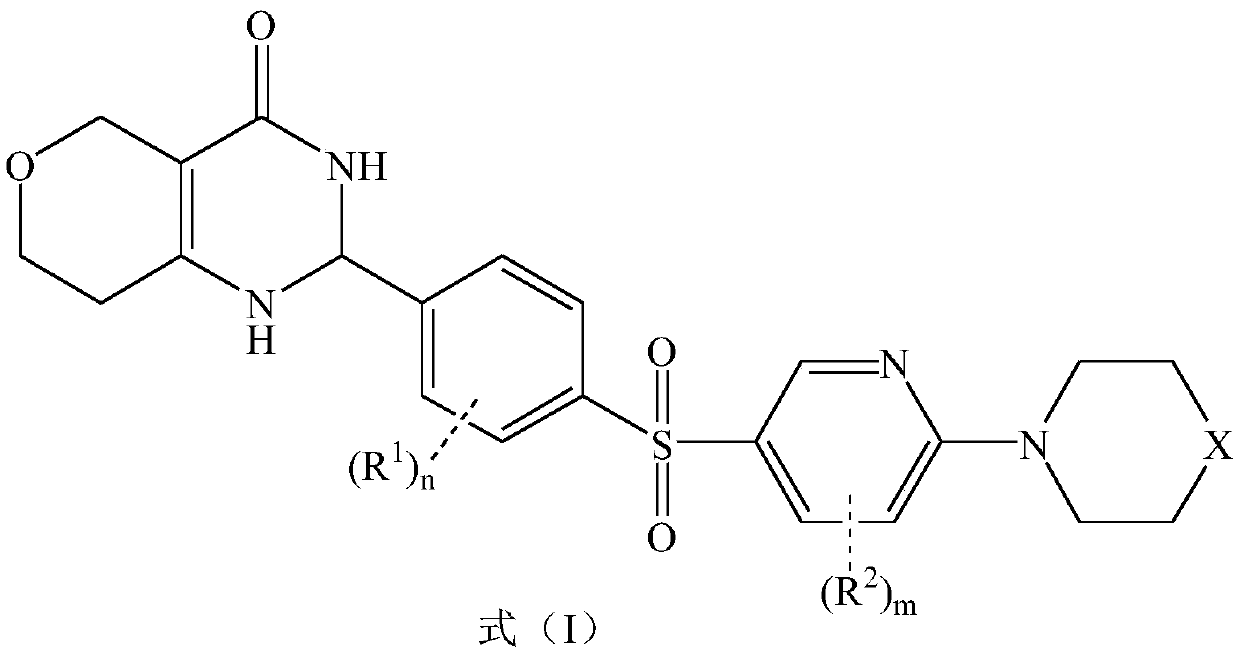
[0024] 2-[3-Methyl-4-(4-piperazin-1-yl-benzenesulfonyl)-phenyl]-1,2,3,5,7,8-hexahydropyrano[4,3 - d] pyrimidin-4-one
[0025] Step I: Preparation of (2-mercapto-pyridin-5-yl)piperazine
[0026] 2-Mercapto-5-chloropyridine (0.725g, 5mmol), piperazine (0.43g, 5mmol) and N,N-diisopropylethylamine (0.65g, 5mmol) were added into DMSO (20mL) solvent, React overnight at 100°C under nitrogen protection. Ethyl acetate (200 mL) and water (100 mL) were added to the system, the layers were separated, and the organic phase was dried over anhydrous sodium sulfate and dried under reduced pressure to obtain 0.7 g of the title compound with a yield of 73.5%.
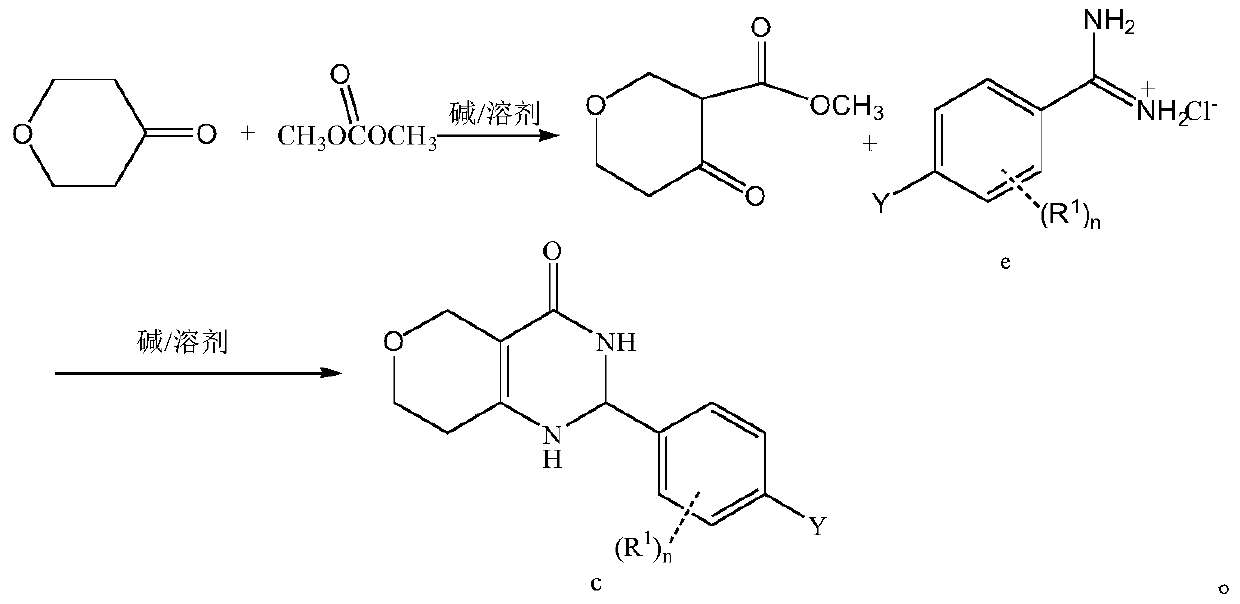
[0027] Step II: Preparation of methyl 4-oxotetrahydro-2H-pyran-3-carboxylate
[0028] NaH (60% by mass, 5 g, 125 mmol) was slowly added to tetrahydrofuran (200 mL) at room temperature, and tetrahydro-4H-pyran-4-one (5.0 g, 50 mmol) and dimethyl carbonate were added after the addition was complete (10 mL, 125 mmol). The temper...
Embodiment 2
[0038]2-[3-Methyl-4-(4-piperidin-1-yl-benzenesulfonyl)-phenyl]-1,2,3,5,7,8-hexahydropyrano[4,3 -d] Preparation of pyrimidin-4-one
[0039] Step I: Preparation of (5-mercapto-pyridin-2-yl)piperidine
[0040] 5-Mercapto-2-chloropyridine (0.725g, 5mmol), piperidine (0.42g, 5mmol) and N,N-diisopropylethylamine (0.65g, 5mmol) were added to DMSO (20mL) solvent, React overnight at 100°C under nitrogen protection. Ethyl acetate (200 mL) and water (100 mL) were added to the system, the layers were separated, and the organic phase was dried over anhydrous sodium sulfate and dried under reduced pressure to obtain 0.62 g of the title compound with a yield of 65.1%.
[0041] Step II: 2-[3-Methyl-4-(4-piperidin-1-yl-phenylmercapto)-phenyl]-1,2,3,5,7,8-hexahydropyrano Preparation of [4,3-d]pyrimidin-4-one
[0042] (5-Mercapto-pyridin-2-yl)piperidine (193mg, 1mmol) prepared in step I and 2-(4-bromophenyl)-3,5,7,8 prepared in step III of Example 1 -Tetrahydro-4H-pyrano[4,3-d]pyrimidi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com