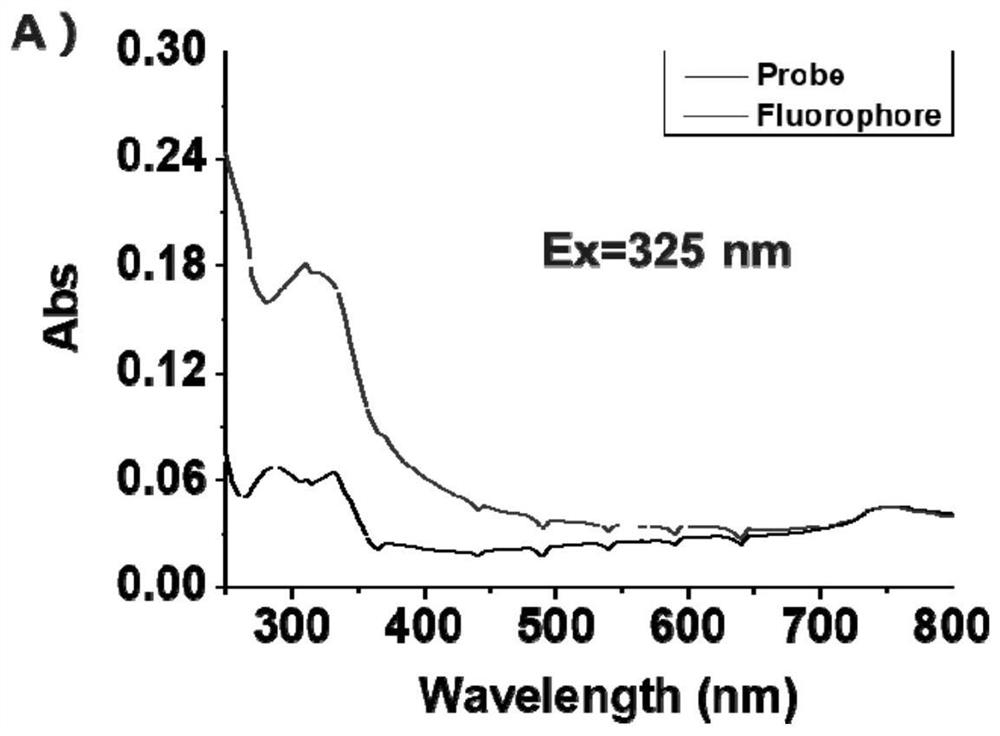
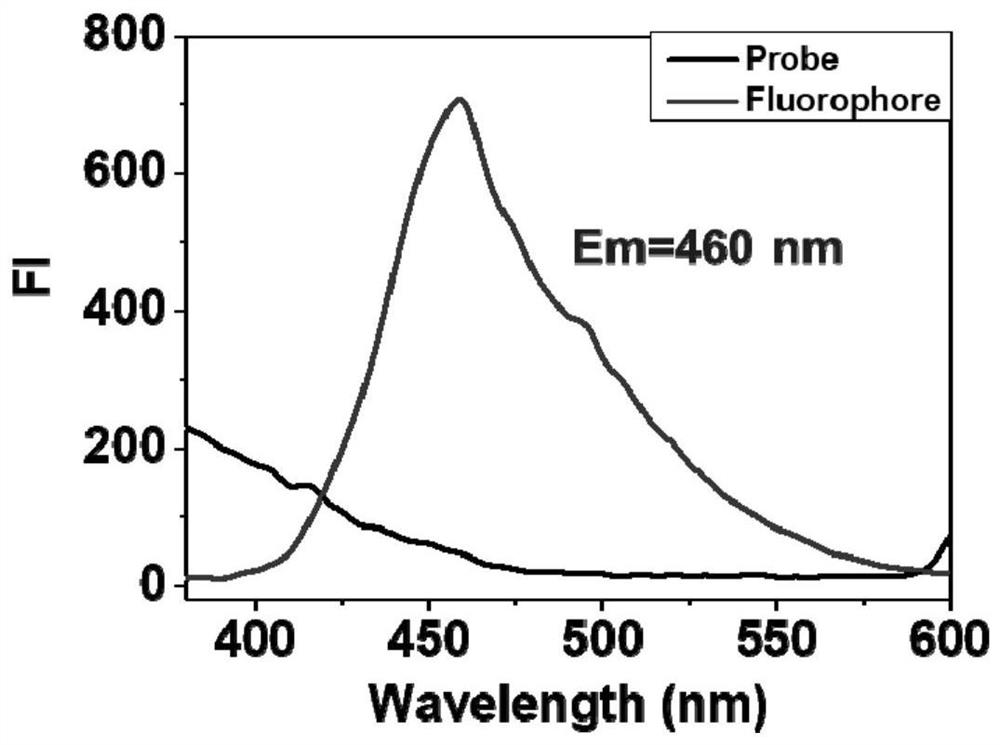
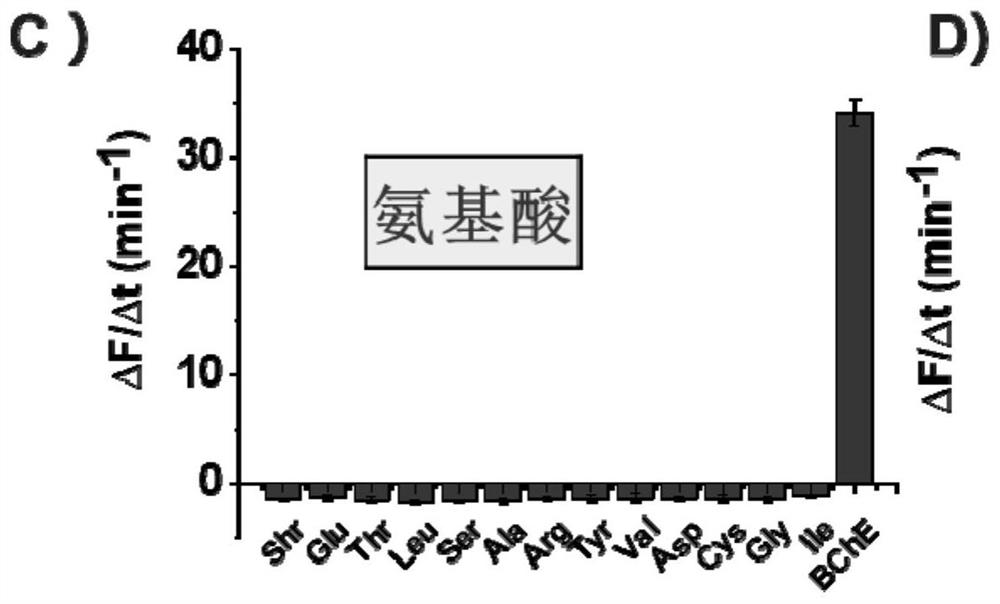
Fluorescent probe for detecting butyrylcholinesterase and its application
A technology for detecting butyrylcholinesterase and butyrylcholine, which is applied in the field of fluorescent probes for detecting butyrylcholinesterase, and can solve the problems of low atom utilization, poor stability of polypeptide fluorescent probes, and storage and transportation conditions. Harsh and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] This example is used to illustrate the method for detecting the activity of butyrylcholinesterase by the compound represented by formula (1) provided by the present invention.
[0077] Dissolve 1 mg of butyrylcholinesterase sample in 100 mM Tris-HCl (containing 10 mM CaCl 2 , pH=7.0) buffer solution and carry out gradient dilution, prepare the butyrylcholinesterase standard solution of different concentrations according to the concentration given in table 1, the butyrylcholinesterase standard solution that configuration obtains is placed in Preheat in a constant temperature water bath at 30°C; at the same time, place a black flat-bottomed 96-well plate in a 30°C incubator to preheat. Subsequently, 40 μL of preheated butyrylcholinesterase standard solution was placed in a preheated black flat-bottomed 96-well plate, and then 120 μL of 100 mM Tris-HCl (containing 10 mM CaCl 2 , pH=7.0) buffer solution, and a negative control group (only 160 μl of buffer solution was adde...
Embodiment 2
[0082] This example is used to illustrate the method for measuring the activities of elastase, butyrylcholinesterase, trypsin and carboxypeptidase using the non-peptide fluorescent probe represented by formula (1).
[0083]
[0084] Each butyrylcholinesterase sample was dissolved in 100mM Tris-HCl (containing 10mM CaCl 2 , pH=7.0) buffer solution and diluted to prepare an enzyme solution with a concentration of 0.01 mg / L; at the same time, place a black flat-bottomed 96-well plate in a 30°C incubator to preheat. Subsequently, 40 μL of the preheated enzyme solution was placed in a preheated black flat-bottomed 96-well plate, and then 120 μL of 100 mM Tris-HCl (containing 10 mM CaCl 2 , pH=7.0) buffer solution, and a negative control group (only 160 μl of buffer solution was added), under light-shielding conditions, 40 μL of the probe with the structure shown in formula (1) was contacted with the enzyme solution and the control group respectively, and used The microplate rea...
Embodiment 3
[0092] This example is used to illustrate the screening method for butyrylcholinesterase inhibitors according to the present invention.
[0093] Dissolve 1 mg of butyrylcholinesterase sample in 100 mM Tris-HCl (containing 10 mM CaCl 2 , pH=7.0) in the buffer solution, the concentration of butyrylcholinesterase is 0.05mg / mL to be tested butyrylcholinesterase standard solution, the butyrylcholinesterase inhibitor tacrine is dissolved in 1mL 100mM Tris-HCl (containing 10mM CaCl 2 , pH=7.0) buffer solution to configure inhibitor solutions with concentration gradients of 0.1, 1, 4, 7, 10, and 20 μM, and dissolve 0.01 mmol of the fluorescent probe with the structure shown in formula (1) in 10 mL of 100 mM Tris-HCl (contains 10mM CaCl 2 , pH=7.0) buffer solution to be configured as a fluorescent probe solution with a concentration of 1 mM, and the butyrylcholinesterase standard solution, butyrylcholinesterase inhibitor tacrine solution and fluorescent probe The solution was prehea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com