Composition containing ursodeoxycholic acid for prevention or treatment of visual impairment
一种熊去氧胆酸、视力障碍的技术,应用在药物组合物,预防或治疗视力障碍的组合物领域,达到促进恢复、消除疼痛和恐惧、抑制发展的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0215] (Example 1) The weight ratio of UDCA and maltodextrin is the clarified aqueous solution of 1:6
[0216] A clear aqueous stock solution of water-soluble UDCA containing native UDCA and low dextrose equivalent water-soluble starch was prepared.
[0217] Specifically, 6.7 g of sodium hydroxide granules were dissolved in 400 ml of purified water. 60 g of UDCA were dissolved in sodium hydroxide solution at room temperature while stirring. 360 g of maltodextrin were slowly added to the clear solution while stirring. Preservatives were then added to the clear solution obtained by sonication at high throughput (750 W, 20 kHz) in an amount suitable for pharmaceutical formulations, and the pH was adjusted by the dropwise addition of HCl. Add purified water and adjust to a total of 1000ml. Filter the clear solution through a suitable filter unit if necessary. This filtration is important to remove impurities from the raw material or to sterilize it, but since the solution is a...
Embodiment 2
[0218] (Example 2) The weight ratio of UDCA and maltodextrin is the clarified aqueous solution of 1:12
[0219] A clear aqueous stock solution of water-soluble UDCA containing native UDCA and low dextrose equivalent water-soluble starch was prepared.
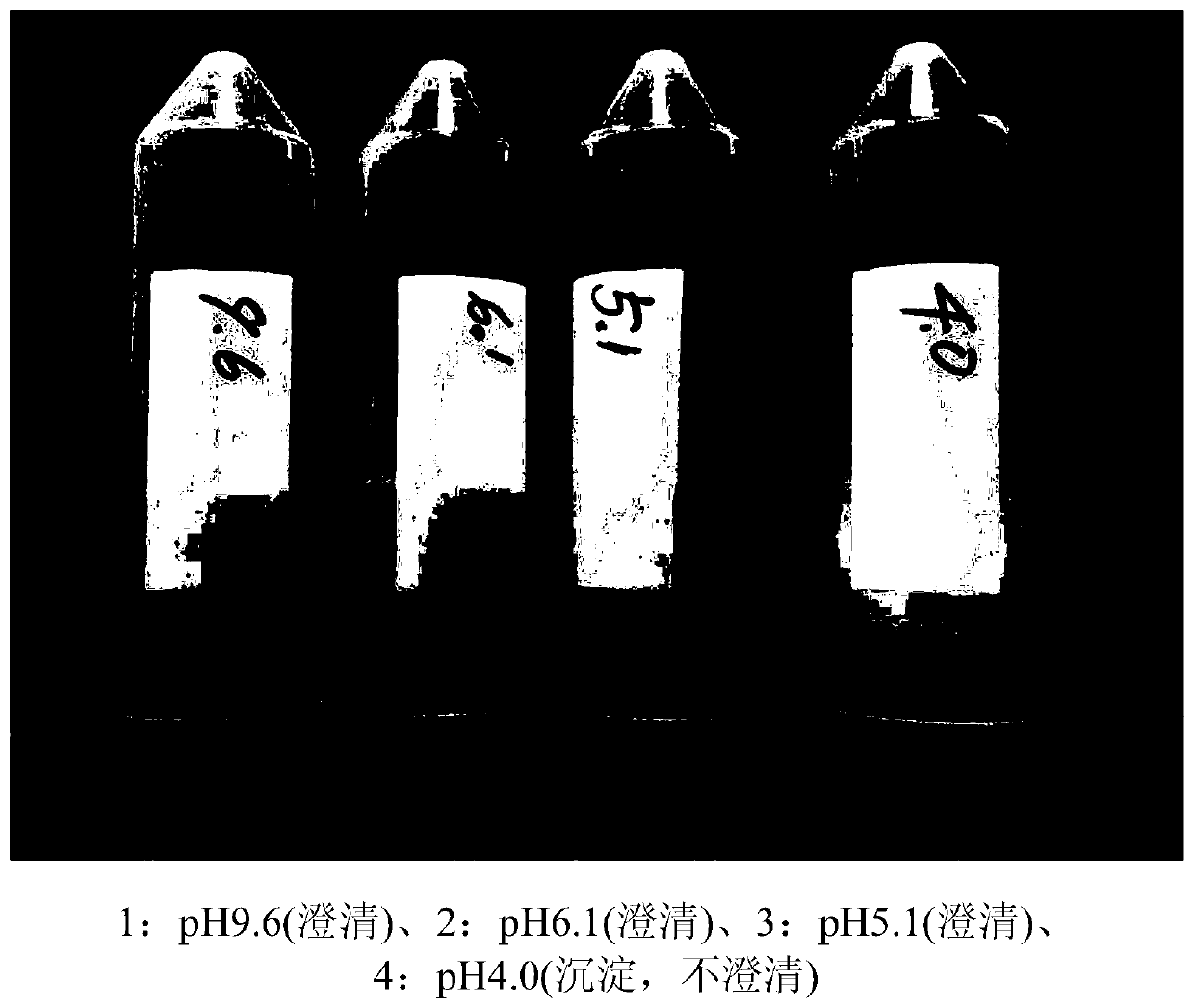
[0220]Specifically, it was prepared according to the same procedure as in Example 1, except that 720 g of maltodextrin, which is a high molecular weight water-soluble starch conversion product, was used per 60 g of ursodeoxycholic acid. As shown in Table 3, the prepared ursodeoxycholic acid solutions formed clear aqueous solutions without any visually visible precipitate at pH 9.6, 7.3, 6.5 and 6.0, but formed a precipitate at pH 5.5.
Embodiment 3
[0221] (Example 3) The weight ratio of UDCA and maltodextrin is the clarified aqueous solution of 1:15
[0222] A clear aqueous stock solution of water-soluble UDCA containing native UDCA and low dextrose equivalent water-soluble starch was prepared.
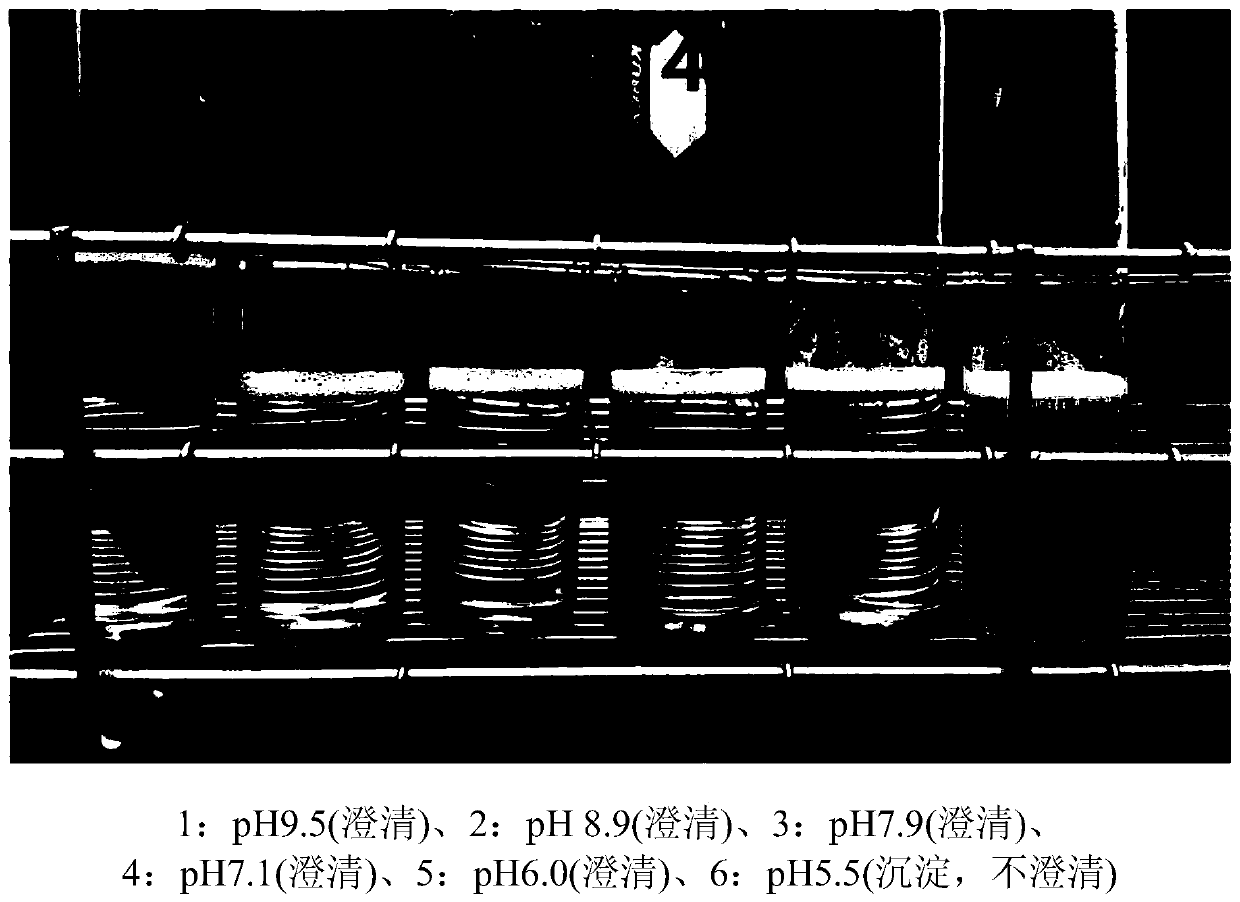
[0223] Specifically, it was prepared according to the same procedure as in Example 1, except that 750 g of maltodextrin, which is a high molecular weight water-soluble starch conversion product, was used per 50 g of ursodeoxycholic acid. Dissolve 5.7 g of sodium hydroxide granules in 400 ml of purified water and use. As shown in Table 3, the prepared ursodeoxycholic acid solutions formed clear aqueous solutions with no visually visible precipitate at pH 9.5, 8.9, 7.9, 7.1 and 6.0, but formed a precipitate at pH 5.5. figure 1 is a graph showing whether a clear aqueous solution of ursodeoxycholic acid was formed at each pH value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com