Method for treating autoimmune disease using cd4 t-cells with engineered stabilization of expression of endogennous foxp3 gene
A gene and heterologous technology, applied in gene therapy, allergic diseases, animal cells, etc., can solve the problems of exacerbation of autoimmune symptoms and lack of stability of pTregs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
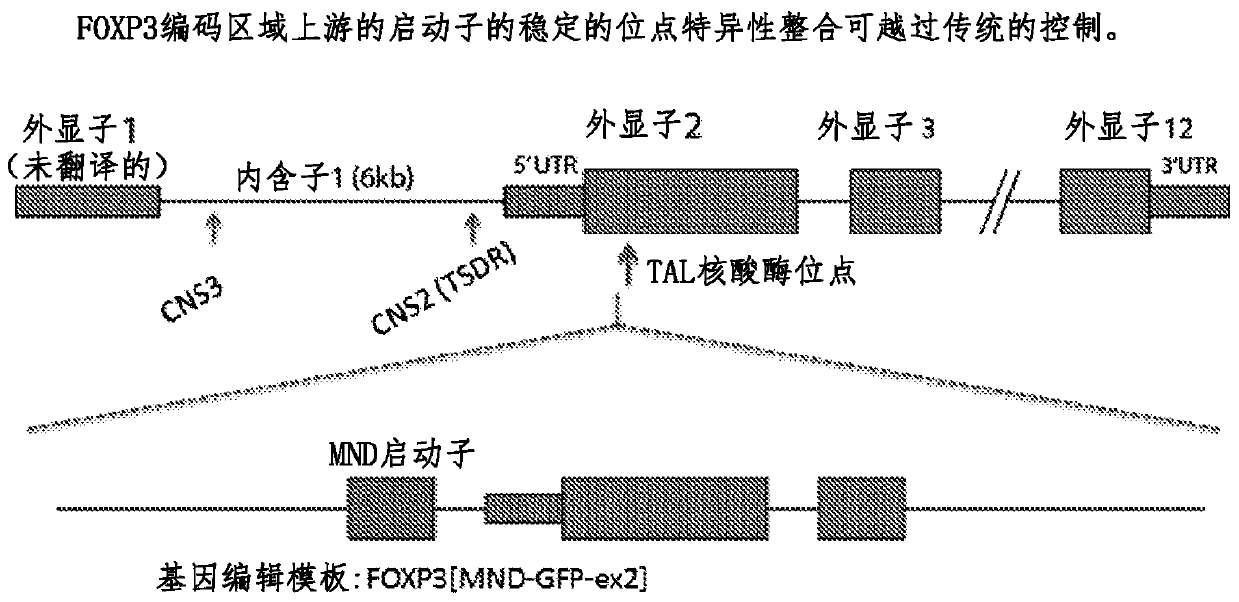
[0093] Alternative Embodiment 1: Gene editing effectively targets the FOXP3 gene and drives high expression of the transgene.
[0094] Such as Image 6 As shown, cells engineered to express GFP under the control of the MND promoter fused to the N-terminus of the endogenous FOXP3 gene showed high levels of GFP-FOXP3 fusion protein expression. Such as Figure 7 As shown, the edited cells display HR-mediated seamless integration at the target site. for Figure 7 In the experiments in , PCR was performed with the indicated primer set (ie corresponding to the position of the red arrow on the figure), and the obtained PCR products were analyzed by agarose gel electrophoresis. PCR using one primer targeting the outside of the template (primer 1 or primer 2) and one primer targeting the inside of the template (primer 3 or primer 4) will only show bands of the correct size if precise targeted integration occurs. The gel panels on the left illustrate the lack of targeted integratio...
Embodiment approach 2
[0099] Alternative embodiment 2: Forced FOXP3 expression produces reg Surface and cytokine phenotypes of T cells
[0100] Figure 10 T showing surface marker and cytokine phenotypes are shown reg Cell cartoons. It is desirable to see whether the edited cells have a specific surface phenotype. Such as Figure 11 As indicated, CD25, CD127, CTLA4 and LAG3 expression of engineered T cells stably expressing FOXP3 were analyzed by flow cytometry. Similar to natural regulatory T cells, the engineered cells were shown to express high CD25, low CD127, and high CTLA4 and Lag3.
[0101] Figure 12 Cytokine expression profiles of edited cells are also shown in . Such as Figure 12 As shown, engineered cells exhibited intracellular cytokine expression in T cells compared to mock-edited T cells. reg Like profile, with low expression of IL2, IL4 and IFN-γ. Such as Figure 13 As shown in the bar graph in , engineered T cells stably expressing FOXP3 showed high expression of IL-10 c...
Embodiment approach 3
[0108] Alternative Embodiment 3: Functional Activity of Edited Cells, Edited Cells Can Inhibit T eff proliferation.
[0109] Figure 16 A schematic diagram of the assay to read out the suppressive activity of regulatory T cells is shown. Combine CFSE-labeled responder cells with edited T regs Or mock-edited cells were mixed and subjected to bead stimulation. The assay is read off by the extent of CFSE dilution at 96 hours.
[0110] Figure 17 show that edited T cells suppress T eff proliferation. Responder cells incubated with mock-edited cells were able to substantially dilute CFSE labeling through their proliferation, whereas responder cells incubated with engineered GFP+ cells remained largely undivided (with a large amount of residual labeling).
[0111] Figure 18 Functional activity of edited cells is shown: using edited T cells from IPEX subjects lacking response to T eff Inhibition of proliferation. use with Figure 17 In the same assay, normal subject contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com