Micro-immunofluorescence detection method without cell loss
An immunofluorescence detection and cell-free technology, which is applied in the biological field, can solve the problems of limiting the application of immunofluorescence technology and difficult identification, and achieve the effects of improving visibility, reducing experimental background, and eliminating cell loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1. A micro-immunofluorescence detection method without cell loss, the following steps are carried out in sequence:
[0107] 1) Sterilize the 0.22um Spin-X Centrifuge Tube with high-pressure steam (pressure of 103.4KPa, temperature of 121°C, sterilization time of 30 minutes);
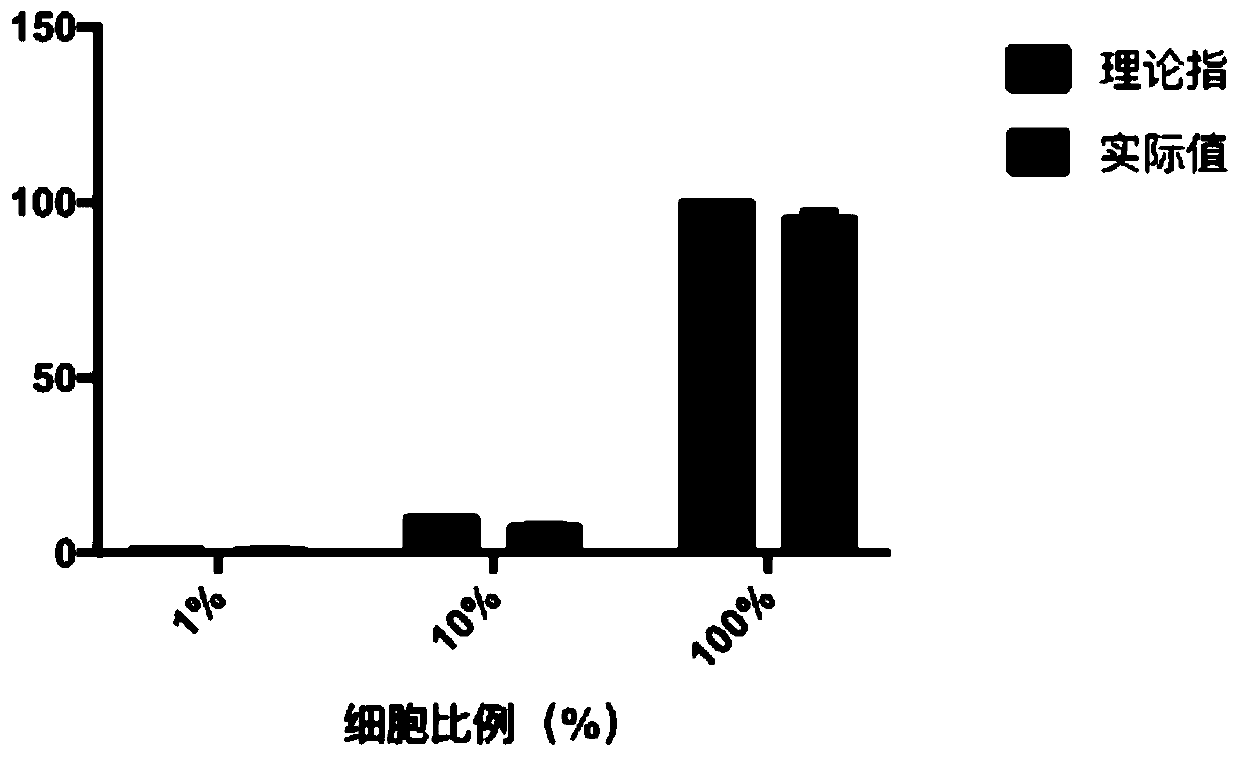
[0108] 2), using a cell counting board to take a certain number of A549 cells and 3T3 cells to mix, respectively to obtain the following experimental groups:
[0109] Experimental group 1: the ratio of A549 cells is 1% (ie, A549 cells: 3T3 cells = 1:99);
[0110] Experimental group 2: the ratio of A549 cells is 10% (ie, A549 cells: 3T3 cells = 1:9);
[0111] Experimental group 3: the proportion of A549 cells is 100% (that is, all are A549 cells);
[0112] The above three experimental groups all meet the following conditions: the total number of cells is 1*10 5 pcs; the volume is 0.5ml;
[0113] The above-mentioned 3 experimental groups were respectively carried out as follows: trans...
Embodiment 2
[0144] Embodiment 2: Compared with Embodiment 1, only step 2 is changed; the rest is the same as Embodiment 1.
[0145] 2) Set up the following experimental groups respectively:
[0146] Experimental group 1 (negative control experiment): 0.5ml of cell culture medium was directly transferred to the sterilized 0.22um Spin-X Centrifuge Tube obtained in step 1);
[0147] Experimental group 2: the adherent cells --- mouse fibroblast 3T3 cells (concentration: 1*10 4 ) 0.5ml is directly transferred to the sterilized 0.22um Spin-X Centrifuge Tube obtained in step 1);
[0148] Experimental group 3: non-adherent cells - human acute leukemia cells HL60 (concentration 1*10 4 ) 0.5ml is directly transferred to the sterilized 0.22um Spin-X Centrifuge Tube obtained in step 1);
[0149] Experimental group 4: non-adherent cells - human acute leukemia cells HL60 (concentration 1*10 4) 0.5ml is transferred to the sterilized 0.22um Spin-X Centrifuge Tube obtained in step 1) and cultivated in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com