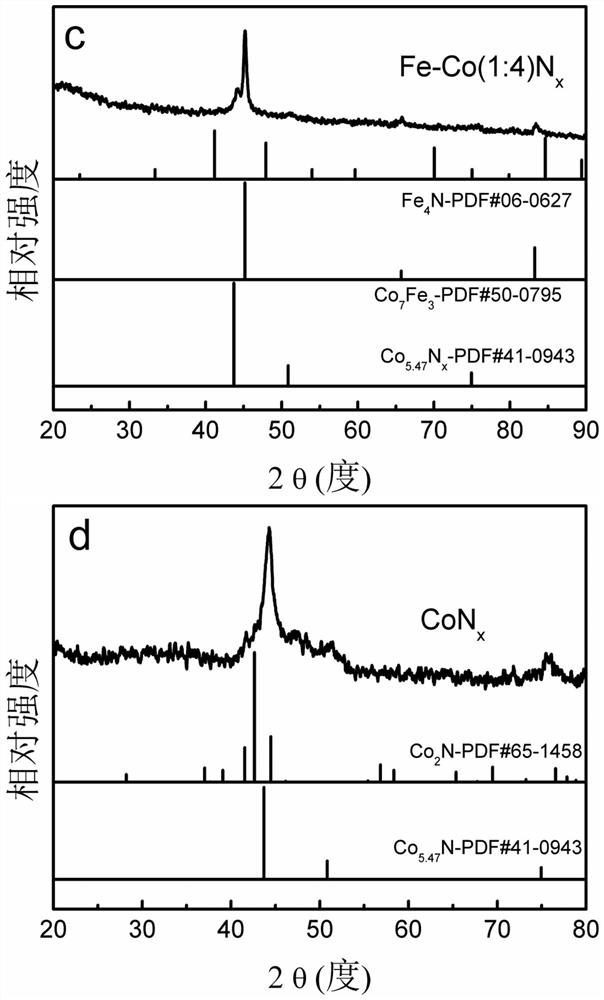
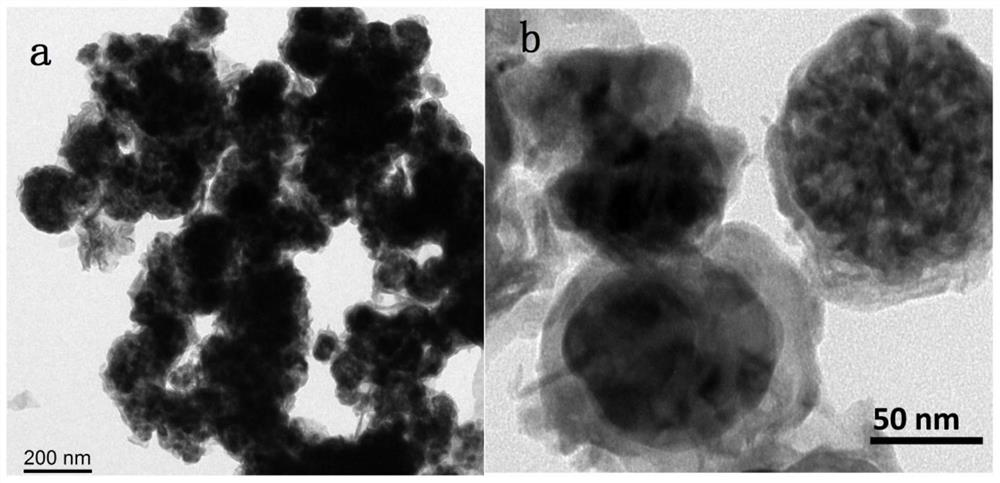
A kind of bimetallic Fe-co nitride electrocatalyst and its preparation method and application
A nitride electro and bimetallic technology, applied in the field of electrocatalysis, can solve the problem of little research on oxygen evolution reaction catalysts, and achieve the effects of improving catalytic oxygen evolution reaction performance, less environmental pollution, high N content and specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Fe-Co(2:1)N x Preparation of electrocatalyst
[0031] Weigh 1.65mmol Co(NO 3 ) 2 .6H 2 O, 3.31mmol Fe(NO 3 ) 3 .9H 2 O was dissolved in 20ml deionized water to make metal precursor solution A; 26mmol excess NaBH was weighed 4 Dissolve in 150ml deionized water to make reducing solution B (molar concentration is 0.17mol / L); at room temperature, under 900 rpm magnetic stirring and Ar gas protection conditions, add solution B dropwise to metal precursor solution A , continue to stir and react for 2h to obtain a black mixture; centrifuge the obtained mixture, wash with deionized water, and vacuum dry at 60°C for 12h to obtain a solid powder; put the solid powder in a tube furnace NH 3 Under the atmosphere, the temperature was raised to 500°C at a heating rate of 8°C / min, kept for 2 hours, and the bimetallic Fe-Co nitride electrocatalyst was obtained after cooling, which was recorded as Fe-Co(2:1)N x electrocatalyst.
[0032] For the bimetallic Fe-Co(2:1)N...
Embodiment 2
[0034] Example 2: Fe-Co(1:3)N x Preparation of electrocatalyst
[0035] Weigh 4.59mmol Co(NO 3 ) 2 .6H 2 O, 1.53mmol Fe(NO 3 ) 3 .9H 2 O was dissolved in 30ml deionized water to make metal precursor solution A; then weigh 26mmol excess NaBH 4 Dissolve in 100ml deionized water to make reducing solution B (molar concentration is 0.26mol / L); at room temperature, under 700 rpm magnetic stirring and Ar gas protection conditions, add solution B dropwise to metal precursor solution A , continue to stir and react for 1h to obtain a black mixture; centrifuge the resulting mixture, wash with deionized water, and vacuum dry at 60°C for 12h to obtain a solid powder; put the solid powder in a tube furnace NH 3 Under the atmosphere, the temperature was raised to 500°C at a heating rate of 5°C / min, kept for 2 hours, and after cooling, a bimetallic Fe-Co nitride electrocatalyst was obtained, which was denoted as Fe-Co(1:3)N x electrocatalyst.
[0036] For the Fe-Co(1:3)N prepared by ...
Embodiment 3
[0039] Example 3: Fe-Co(1:5)N x Catalyst preparation
[0040] Weigh 4.59mmol Co(NO 3 ) 2 .6H 2 O, 0.92mmol Fe(NO 3 ) 3 .9H 2 O was dissolved in 40ml deionized water to make metal precursor solution A; then weigh 26mmol excess NaBH 4 Dissolve in 50ml deionized water and make reducing solution B (molar concentration is 0.52mol / L); At room temperature, 900 rpm magnetic stirring and N 2 Under gas-protected conditions, solution B was added dropwise to metal precursor solution A, and the reaction was continued for 0.5 h to obtain a black mixture; the obtained mixture was centrifuged, washed with deionized water, and vacuum-dried at 80°C for 8 hours to obtain a solid powder; Put the solid powder in the tube furnace NH 3 Under the atmosphere, the temperature was raised to 500°C at a heating rate of 5°C / min, kept for 2 hours, and the bimetallic Fe-Co nitride electrocatalyst was obtained after cooling, which was recorded as Fe-Co(1:5)N x electrocatalyst.
[0041] For the Fe-Co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com