The synthetic method of hydroxamic acid compound
A synthesis method and compound technology, applied in the direction of organic chemistry, etc., can solve the problems of high reaction temperature, complex post-processing, and many wastes, and achieve the effects of high molecular concentration, simple post-processing, and avoiding environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Add 24.55g of benzoic acid (99.5% in content) and 21.05g of acetic anhydride (97% in content) into a ball mill, grind for 5 minutes, then add 14.11g of hydroxylamine hydrochloride (98.5% in content), and continue grinding for 45 minutes to obtain a white solid The product is the target collector product. Through analysis and detection, the content of benzyl hydroxamic acid is 51.82%, and the yield of benzyl hydroxamic acid based on benzoic acid is 95.81%.
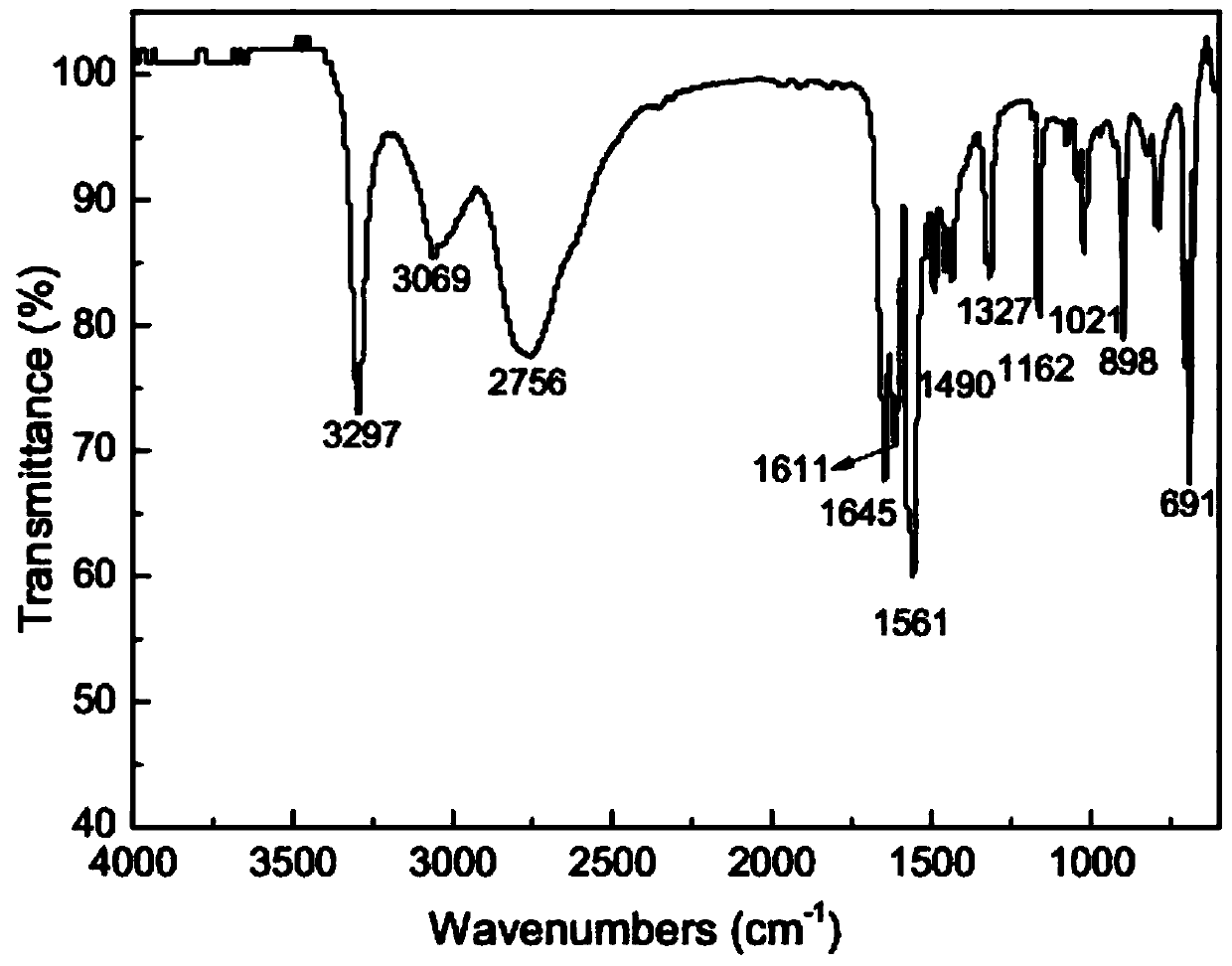
[0067] The product was characterized after being separated and purified by column chromatography, and the infrared spectrum of benzohydroxamic acid was as follows: figure 1 As shown, its main characteristic peaks are (cm -1 ): 3297 belongs to the N-H stretching vibration peak, 3069 belongs to the C=C-H stretching vibration peak on the benzene ring, 2756 belongs to the O-H stretching vibration peak, 1645 and 1611 belong to the -HN-C=O vibration peak, 1561 belongs to the -C=N- Stretching vibration peaks, 1490 and 145...
Embodiment 2
[0071] Add 24.55g of benzoic acid (99.5% in content) and 21.05g of acetic anhydride (97% in content) into a ball mill, grind for 5 minutes, then add 14.11g of hydroxylamine hydrochloride (98.5% in content), and continue grinding for 45 minutes to obtain a white solid The product was washed three times with 50ml of distilled water, filtered, and the filter cake was vacuum-dried at 50°C to obtain a pure white solid product, which was the target collector product. Through analysis and detection, the content of benzohydroxamic acid is 90.55%, and the yield of benzoic acid-based benzohydroxamic acid is 91.79%.
Embodiment 3
[0073] 24.55g benzoic acid (content is 99.5%) and 41.68g dicyclohexylcarbodiimide (DCC) (content is 99%) add ball mill jar, grind 5min, then add 14.11g hydroxylamine hydrochloride (content is 98.5%), After continuing to grind for 45 minutes, a white solid product was obtained, which was the target collector product. Through analysis and detection, the content of benzohydroxamic acid is 37.04%, and the yield of benzoic acid-based benzohydroxamic acid is 94.74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com