A kind of synthetic method of d-cycloserine
A synthesis method and cycloserine technology, applied in the direction of organic chemistry and the like, can solve the problems of low final yield, expensive raw materials, large energy consumption, etc., and achieve the effects of simple process, easier industrialization, and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
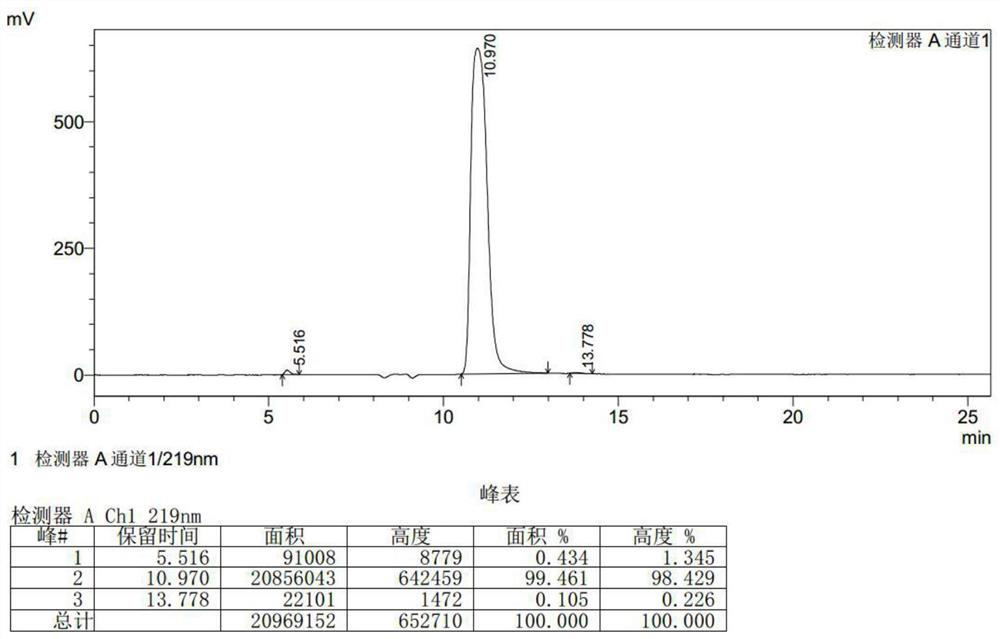
[0038] Put 174g of R-3-chloroserine methyl ester hydrochloride in methanol, add 38ml of hydrochloric acid, then add 87.6g of acetone oxime, heat up to 45°C for 1 hour, cool down to 25°C, use sodium methoxide to maintain the pH to 8-9, React for 2 hours, filter to remove salt, and concentrate the mother liquor to a small volume to obtain the product D-cycloserine. (The quality of the obtained product D-cycloserine is 93.95g, the purity is 99.461%, and the yield is 91.53%).
Embodiment 2
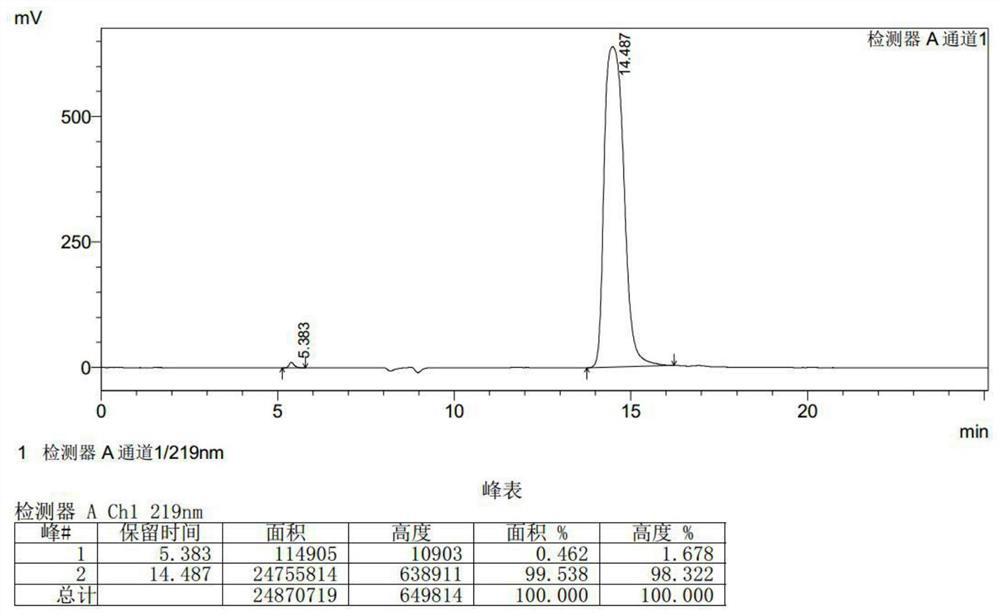
[0040] Put 174g of R-3-chloroserine methyl ester hydrochloride in methanol, add 44ml of hydrochloric acid, then add 87.6g of acetone oxime, heat up to 55°C for 1 hour, cool down to 10°C, use sodium methoxide to maintain the pH to 8-9, React for 3 hours, filter to remove salt, and concentrate the mother liquor to a small volume to obtain the product D-cycloserine. (The quality of the obtained product D-cycloserine is 96.11g, the purity is 99.538%, and the yield is 93.71%).
Embodiment 3
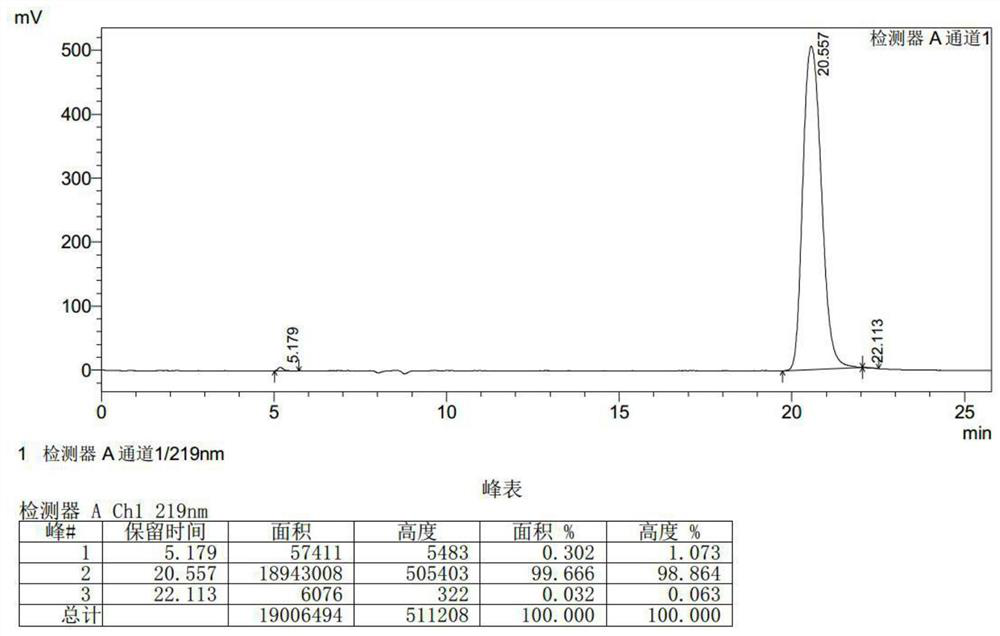
[0042] Put 174g of R-3-chloroserine methyl ester hydrochloride in methanol, add 46ml of hydrochloric acid, then add 87.6g of acetone oxime, raise the temperature to 65°C for 1 hour, cool down to 5°C, and maintain the pH to 8-9 with sodium methoxide. React for 5 hours, filter to remove salt, and concentrate the mother liquor to a small volume to obtain the product D-cycloserine. (The quality of the product D-cycloserine obtained is 96.55g, the purity is 99.666%, and the yield is 94.25%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com